teaser
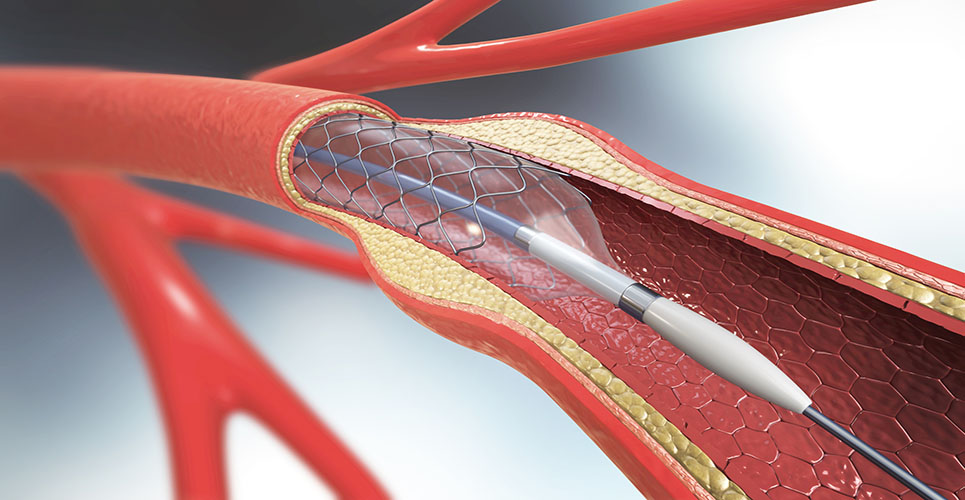
A report commissioned by the Health Technology Assessment has reviewed the clinical and cost-effectiveness of the use of drug-eluting coronary artery stents in percutaneous coronary intervention (PCI) in patients with coronary artery disease (CAD).
The review, on behalf of the National Institute for Health and Clinical Excellence, compared the use of drug-eluting stents (DES) versus bare metal stents (BMS) and drug-eluting stents of different design (DES versus DES).
Following the studies, the HTA concluded that the use of DES is best targeted at the subgroups of patients with the highest risks of requiring re-intervention and could be considered cost-effective in only a small percentage of such patents.
The HTA said that this was similar to the conclusion of its previous assessment in 2003 and added that trials of DES compared with new generation BMS and with DES would be useful, as would further evaluation of newer BMS in combination with drug administration.
Regarding the continuation of antiplatelet therapy post-procedure, the HTA said: “The question of follow-up medication post-PCI was explored in view of the current lack of consensus on the period of preventive anti-platelet therapy necessary to avoid later thrombosis: suggested periods range between three months and lifetime, and evidence that risks may be greater after DES implantation has led to suggestions that a longer treatment with clopidogrel after DES use may be needed.
“Our clinical guidance indicated that making this distinction in practice would be difficult and that a common follow-up period of, for example, 12 months is more realistic. With the same treatment for DES and non-DES patients, there is no incremental cost and it has been omitted from the model. This approach tends to favour the cost-effectiveness of DES.”