teaser
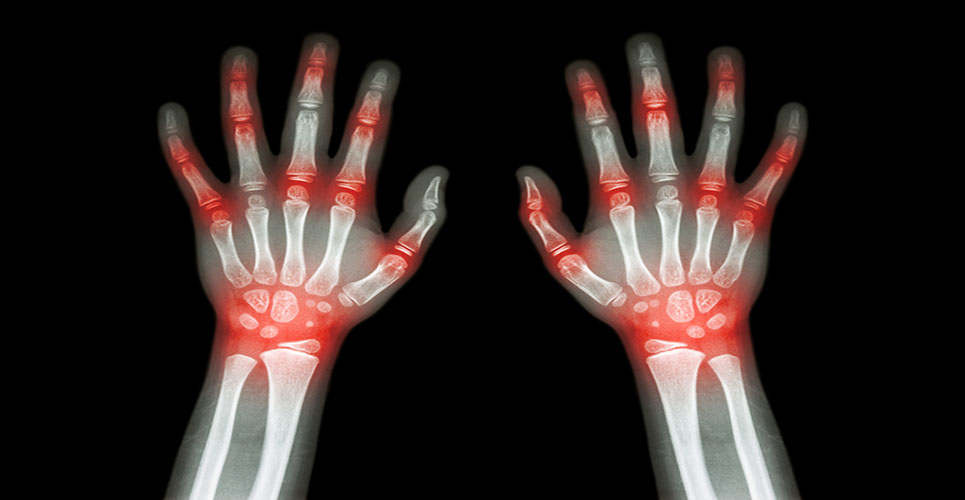
An experimental arthritis drug has met safety and efficacy goals in a 16-week preliminary trial.
The drug, a molecule known as BMS-945429/ALD518 developed by Bristol-Myers Squibb and Alder Biopharmaceuticals, targets a protein called interleukin-6 and was tested in 132 patients suffering from rheumatoid arthritis (RA) that was inadequately controlled with methotrexate.
Symptoms appeared to improve after week 12, and after 16 weeks, 75%-82% of patients had seen at least a 20% improvement in their symptoms.
By comparison, only 36% of those receiving placebo experienced an improvement in symptoms after the same amount of time.
However, four patients (17%) were forced to drop out of the study after experiencing increased levels of liver enzymes, the companies said.
The findings of the study will be presented at the Annual Congress of the European League against Rheumatism on Friday, the companies said.
Rheumatoid arthritis is an auto-immune disease which affects 0.8% of the adult population, causing severe disability and chronic pain. Symptoms include joint swelling, stiffness, fatigue and weight loss.
It is a systemic disease which means that it can affect the whole body and internal organs such as the lungs, heart and eyes.
Copyright Press Association 2010
For more information, please click the links below: