Improving safety from pharmacy to every point of care was the theme of the BD-supported satellite symposium convened on 23 March 2017 at the 22nd EAHP Congress in Cannes, France.
Presentations focused on:
Improving safety from pharmacy to every point of care was the theme of the BD-supported satellite symposium convened on 23 March 2017 at the 22nd EAHP Congress in Cannes, France.
Presentations focused on:
- Reducing risk of exposure to contamination inside isolators during the preparation of injectable antineoplastic drugs
- Use of an integrated software system to reduce medication errors, enhance patient safety and improve compounding workflow
- Integration of an automated dispensing system cabinet with robotic storage and dispensing to save time in delivery to wards, decrease the incidence of inaccurate delivery to the wards and increase patient safety
- Enhancing patient safety in intensive care units by implementation of a comprehensive programme to reduce errors in the intravenous administration of medication
Decreasing the risk of contamination in isolators
The first description of the risk of exposure to antineoplastic drugs was published by Falck in the Lancet in 1979 (Falck K, et al. Lancet 1979;1(8128):250-1), when he and his colleagues reported that unprotected nurses who worked in an environment in which hazardous drugs were prepared and administered had high levels of mutagenic substances in their urine as compared with non-exposed nurses. Patients receiving the antineoplastic drugs were also reported to have high levels (relative to control) of antineoplastic drugs in their urine.
Sources of contamination with hazardous drugs are widely reported and include the external surfaces of vials, the inside surfaces of biosafety cabinets/isolators, preparations sent to the wards and various locations in the wards (including seats on which patients sit and toilets where drugs and/or metabolites are eliminated through the excreta).
A number of recommendations for the safe handling of hazardous drugs have been published in North America and in Europe, the first in 1981 by hospital pharmacist associations. The first European recommendations were published in 2007. Recommendations include those from the Occupational Safety and Health Administration, the National Institute for Occupational Safety and Health, the American Society of Health-System Pharmacists (ASHP), the Oncology Nursing Society, the Pharmaceutical Inspection Co-operative Scheme and the International Society of Oncology Pharmacy Practitioners (ISOPP). Some national guidelines or standards may also be applicable depending on the country.
Current recommendations include:
- The wearing of personal protective equipment for all operators in contact with antineoplastic drugs
- Compounding with laminar air-flow hoods/barrier isolators
-
Using specific devices (ISOPP), specifically:
- Class I: to protect the handler from the outside of the vial/ampoule
- Class II: to protect the operator during drug preparation
- Class III: to protect the patients during the administration of cytotoxic drugs
Nicolas Simon described the challenges his institution faced when moving from an older workplace environment consisting of isolators with drug storage inside and use of needles and double-canal spikes, to a new compounding unit, without disrupting the delivery of 40,000 preparations annually. Key to the project was the adoption of a closed system transfer device (CSTD) that could ‘… mechanically prohibit the transfer of environmental contaminants into the system and the escape of hazardous drug or vapour concentrations from the system’ (ASHP, 2006).
In vitro studies have shown that BD PhaSeal™ was the only one of the CSTDs that did not release vapour of titanium oxide into the environment. Despite the availability of many in vitro studies, only those carried out in real situations may truly evaluate the devices. Studies in North America of contamination inside biosafety cabinets before and after implementation of BD PhaSeal have shown a significant decrease in the number of positive samples in the case of cyclophosphamide and ifosfamide, and in the case of cyclophosphamide, a decrease by a factor of ten in the amount of drug retrieved from the surfaces. The results varied between pharmacies.
To test BD PhaSeal against standard devices for the decontamination of isolators, the Lille group conducted a six-month study in two isolators, for which contamination results were not revealed to the pharmacy team throughout the study. It showed that, with more than 20,000 preparations, there was:
- An average 50% decrease of contamination for the ten drugs tested when BD PhaSeal was used versus standard decontamination procedures
- At least a 50% decrease in contamination on gloves, window and worktop for BD PhaSeal versus standard decontamination
- An up to 72% decrease in cumulative drug amount on surfaces (gloves, window and worktop)
Tests comparing CSTDs with standard cleaning processes in ensuring isolator glove, window and worktop sterility showed variability in reducing contamination.
Reducing the potential for further residual contamination within the isolators was investigated in a second three-month study comparing before and after cleaning with control. In this study, adding a specific decontamination procedure to the use of a CSTD showed a significant reduction in contamination in the intervention group.
Continuing the analysis of isolator contamination, it was concluded that the use of BD PhaSeal significantly resolves the chemical contamination with antineoplastic drugs, and that the combination of a CSTD plus a decontamination process involving sodium dodecyl sulfate and isopropanol further reduces contamination levels.
Reducing medication errors
The good practice guide on recording, coding, reporting and assessing medication errors (EMA/762563/2014) defines a medication error as ‘unintended failure in the drug treatment process that leads to, or has the potential to lead to, harm to the patient’.
They are responsible for millions of adverse events worldwide and they represent a huge preventable cost in terms of hospital admission, unused medication and litigation.
Types of medication error made in drug reconstitution and administration fall into five categories (wrong medication, wrong dose, wrong route of administration, wrong dosing time and wrong patient) and are given in Table 1.
Table 1. Types of medication error
| Type | Definition |
| Prescribing error | Incorrect drug selection (based on indications, contraindications, known allergies, existing drug therapy, and other factors), dose, dosage form, quantity, route, concentration, rate of administration, or instructions for use of a drug product ordered or authorized by physician (or other legitimate prescriber); illegible prescriptions or medication orders that lead to errors that reach the patient |
| Omission error | The failure to administer an ordered dose to a patient before the next scheduled dose, if any |
| Wrong time error | Administration of medication outside a predefined time interval from its scheduled administration time (this interval should be established by each individual health care facility) |
| Unauthorised drug error | Administration to the patient of medication not authorized by a legitimate prescriber for the patient |
| Improper dose error | Administration to the patient of a dose that is greater than or less than the amount ordered by the prescriber or administration of duplicate doses to the patient, i.e. one or more dosage units in addition to those that were ordered |
| Wrong dosage – form error | Administration to the patient of a drug product in a different dosage form than ordered by the prescriber |
| Wrong drug – preparation error | Drug product incorrectly formulated or manipulated before administration |
| Wrong administration – technique error | Inappropriate procedure or improper technique in the administration of a drug |
| Deteriorated drug error | Administration of a drug that has expired or for which the physical or chemical dosage-form integrity has been compromised |
| Monitoring error | Failure to review a prescribed regimen for appropriateness and detection of problems, or failure to use appropriate clinical or laboratory data for adequate assessment of patient response to prescribed therapy |
| Compliance error | Inappropriate patient behaviour regarding adherence to a prescribed modification regimen |
| Other medication error | Any medication error that does not fall into one of the above predefined categories |
| ASHP guidelines on preventing medication errors in hospitals. Am J Hosp Pharm 1993;50(2):305-14 | |
As emphasised by Ülle Helena Meren, while it is unrealistic to try to eliminate errors, it is certainly possible to minimise them.
At her Tallinn institute, oncology drug preparation was introduced in 2007; between 2007 and 2012, nurses prepared and administered cytotoxics in the same day care ward, in a biosafety hood, using a CSTD (since 2010).
In 2012, pharmacy technicians started preparing cytotoxics in a compounding centre, in a Class A isolator, using CSTDs and BD Cato™. Between 2012 and 2016, the number of annual preparations has risen from just over 700 to 20,000.
The benefits of the BD CatoTM system have been four-fold: increased patient safety, a reduction in medication preparation time (35%), improved management of left-over product (and therefore cost savings) and a reallocation of nurses’ time from preparation to patient care.
The computerised physician order entry functionality improved patient care by reducing transcription errors, automatically calculating doses, gravimetrically checking the preparation and automatically documenting the preparation. Patient safety was further enhanced by a reduction in microbiological contamination and spillage in both pharmacy and ward.
Data labels that carry a unique preparation and patient identification number reduce the risk of administering the wrong medication and gravimetric double-checking prohibits a preparation from proceeding unless the amount of medicine in the syringe is within the 3% limit of the calculated dose.
The Tallinn group is about to start a pilot study on the use of bedside scanning, which would double-check that patients are receiving the right medication.
On a closing note, Meren reminded her audience that, while technology can make a huge difference in smarter and safer working practices, skilled staff are at the heart of successful implementation.
Pioneer in medication automation
The Spital STS AG hospital in Thun, Switzerland offers a case study of how medication automation in the pharmacy can be linked to medication automation on the ward to achieve greater safety and smarter resource allocation.
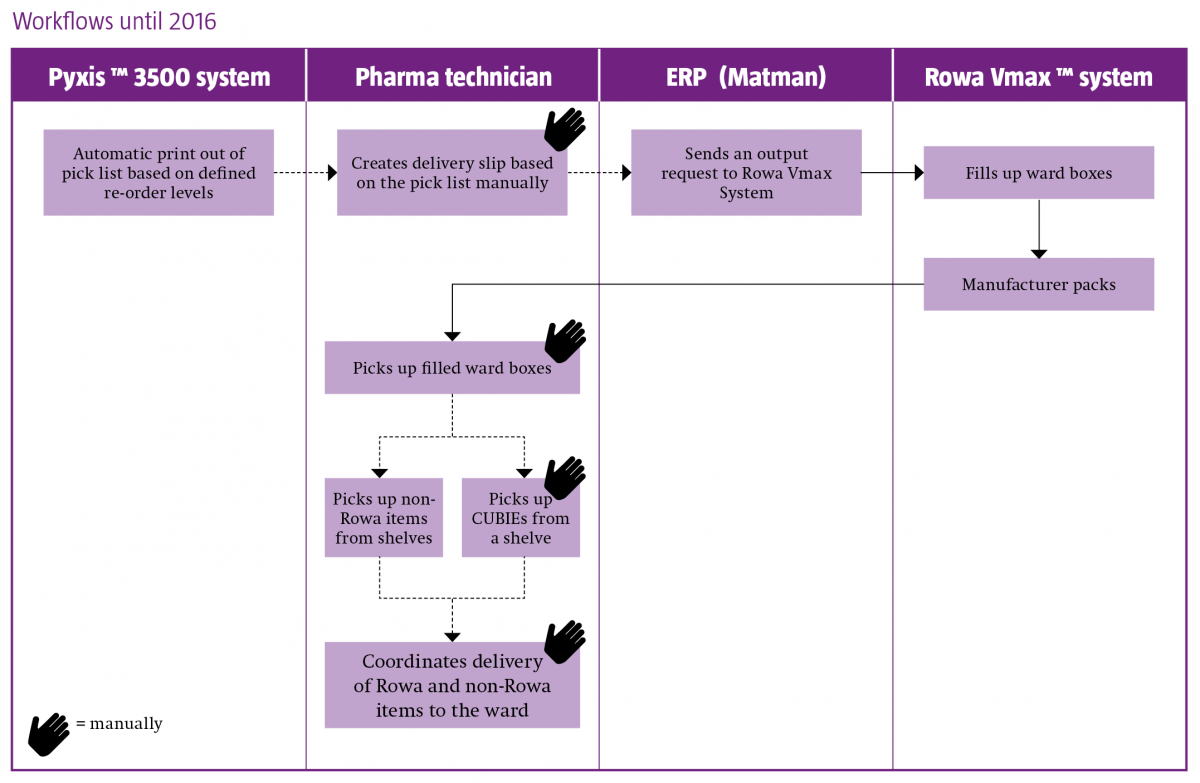
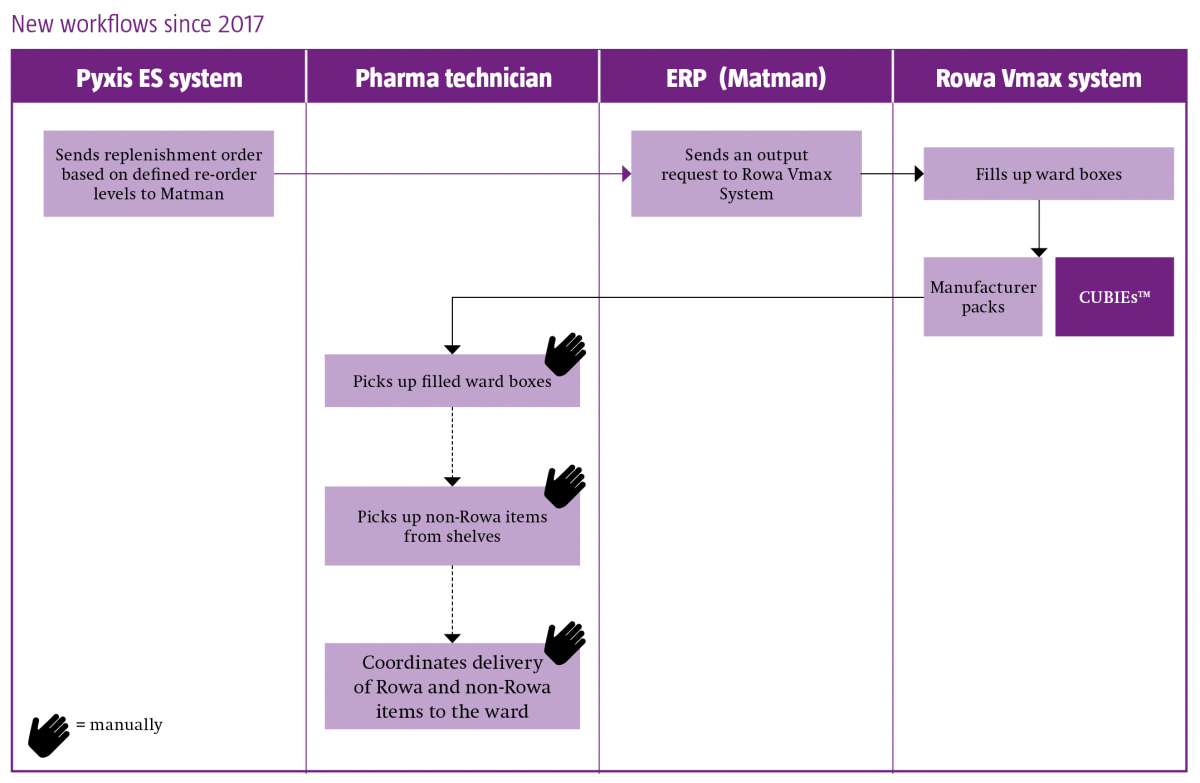
The components of their system landscape are automated dispensing cabinets (Pyxis MedStation™ ES system) on all wards, robotic storage and dispensing (Rowa Vmax™ system) in the pharmacy, computerised physician order entry software (Phoenix™) and enterprise resource planning (ERP) software (Nexus Matman).
Christina Seemann outlined how the goal of integrating ward and pharmacy automation began in 2003, with the decision to implement Pyxis™ on all wards and to connect to Phoenix. Ten years later came the implementation of Rowa Vmax, and the intention to link to Pyxis in a three-stage project:
- Upgrade all Pyxis 3500 stations to Pyxis MedStation ES systems
- Implement interfaces between the ERP system and Pyxis MedStation ES, and between the Pyxis MedStation ES and Rowa Vmax system
- Implementation of the Smart CUBIE™ system (enabling pre-filling of CUBIEs in the pharmacy and storage in Rowa Vmax)
The project was completed in January 2017.
Changes in workflow resulting from the implementation and integration of the medicines automation systems are best illustrated below, and highlight the following benefits:
- A fully automated supply chain
- A decrease in inaccurate delivery to the ward
- Enhanced patient safety
-
An up to 75% time reduction (as observed in the two months since project completion) in the preparation of ward deliveries, enabling the reallocation of staff to the support of nurses and physicians in the provision of enhanced patient-centred care.
Mistakes happen
The challenge posed by medication errors in anaesthesiology has been known for the past 40 years: 25% of all preventable mishaps are caused by drug administration and intravenous (IV) apparatus misuse (Cooper JB, et al. Anesthesiology 1978;49(6):399-406). A number of studies since have quantified the problem:
- One in 20 perioperative medication administrations, and every second operation, have resulted in a medication error/adverse event; more than one-third of these errors have led to observed patient harm (Nanji KC, et al. Anesthesiology 2016;124(1):25-34)
- An error rate of nearly 50% has been reported in the IV drug preparation and administration in a German hospital (Taxis K & Barber N. Eur J Clin Pharmacol 2004;59(11):25-34)
- The intravenous administration of medication represents a common pattern of weakness in patient safety in intensive care units (Valentin A, et al. BMJ 2009;338:b814)
The first step in the approach to pull a conservative clinician environment towards safer practices in the Brandenburg Heart Centre, Germany, presented here by Georg Fritz, was the adoption of a critical incident reporting system (CIRS) that was anonymous, confidential and non-punitive. This process was subsequently endorsed by the World Health Organization and was followed by mandatory uptake in Germany.
Only 40% of German hospitals have an electronic data and order system, and the second step in the process for greater patient safety is the introduction of a patient data management system.
The third step is the adoption of standardised colour coding of IV drugs.
In summary, implementation of the safety programme is defined by the following landmarks:
- Introduction of a standard infusion smart pump, Alaris™ Plus Guardrails™ – all infusion pumps in the hospital are identical and a pump remains with a critically ill patient from surgery to the intensive care unit (2012). These smart pumps have a Dose Error Reduction System (DERS), a software program that sits inside the infusion device and recognises when a deviation from agreed pre-set limits has been attempted.
- Adoption of CIRS (2012)
- Uniformity of labelling (2013)
- Standardised infusion pump protocol, with one smart drug library used throughout the hospital (2014)
-
Implementation of two key elements (2016/17):
i. Alaris™ Communication Engine Platform, an enterprise software solution, enabling uniform infusion protocol throughout the hospital thanks to the remote deployment of the drug library to the infusion pumps while downloading centrally the continuous quality improvement data.
ii. A CQI analytical auditing tool, contributing to a continuous improvement of infusion practice and reduction of medication errors.
Conclusion
Technical innovation enables the safer preparation and administration of hazardous drugs, reduced medication errors, enhanced patient safety and patient-centred staff allocation. Cultural change, senior-level champions and skilled staff are key to successful implementation.
Satellite symposium speakers
Nicolas Simon
Clinical Pharmacist and Assistant Professor of Clinical Pharmacy, Pharmacy Institute, University Hospital of Lille, France
Ülle Helena Meren
Head of Pharmacy, East Tallinn Central Hospital, Estonia
Christina Seemann
Pharmacist, Spital STS AG, Thun, Switzerland
Georg Fritz
Head of the Department for Anaesthesiology, Intensive Care Medicine and Pain Therapy, Immanuel Klinikum Bernau Brandenburg Heart Centre, University Hospital Brandenburg Medical School, Germany