teaser
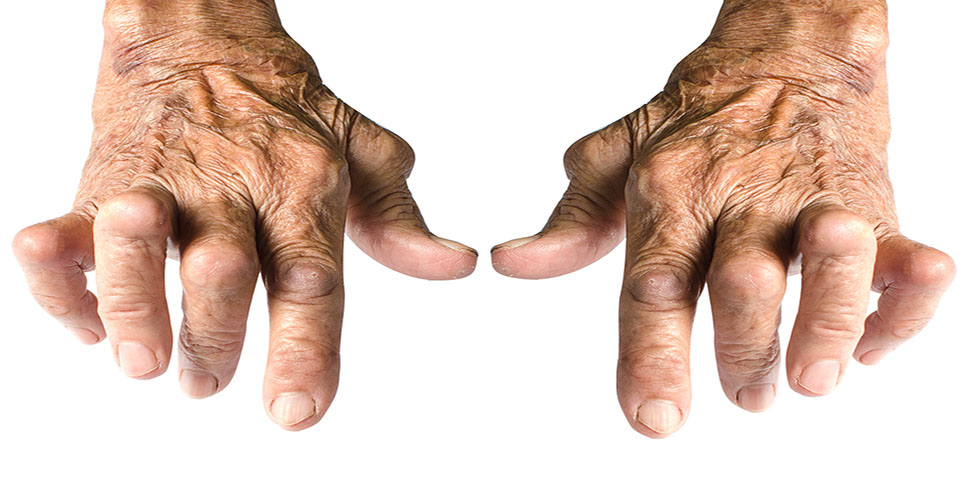
Potentially life-changing drugs are being denied to about 60,000 UK sufferers of rheumatoid arthritis (RA), according to charities.
A decision by the National Institute for Health and Clinical Excellence (NICE) could leave sufferers with pain and the possibility of long-term disability.
New guidance from NICE says that patients who do not respond to a first course of anti-TNF (tumour necrosis factor alpha inhibitor) treatment will not be allowed to try a second.
But charities said that moving from one therapy to a second or third has been established practice in the UK for years.
The British Society for Rheumatology Biologics Register shows that about 70% of patients will get a good response from a second anti-TNF if the effects of the first start to wane.

Charities will have chance to appeal against the decision before final guidance is issued to the NHS in September.
Ailsa Bosworth, chief executive of the National Rheumatoid Arthritis Society, said the move, combined with a NICE decision in April to reject the drug abatacept, meant effective therapies had been cut from five to two.
She added: “This decision is another nail in the coffin for the treatment of RA in England and Wales.
“NICE are rewriting the rules of RA treatment in this country, ignoring the clinical effectiveness of drugs and ignoring the views of patients and clinicians.”
Copyright PA Business 2008