Bimekizumab is the first monoclonal antibody to inhibit both IL-17A and 1L-17F which are implicated in the development of psoriasis.
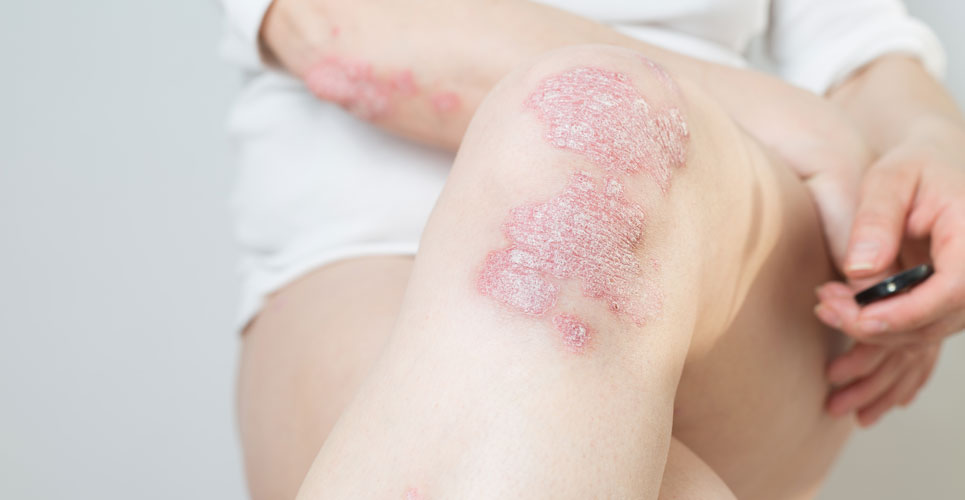
Psoriasis can be defined as a complex, chronic, multifactorial and inflammatory disease which is characterised by enhanced proliferation of keratinocyte cells in the skin, producing silvery/white plaques, typically on extensor surfaces such as the elbows, knees and lower back. The incidence of psoriasis varies across the world and in a recent study, the incidence varied from 30.3 per 100,000 person years in Taiwan to 321 per 100,000 person years in Italy. Fortunately, most people with psoriasis have mild disease and one study of over 9,000 patients, found that just over half (51.8%) had mild disease although 35.8% and 12.4% had moderate and severe disease respectively. Patients with moderate to severe disease are increasing treated with biological agents, in particular, monoclonal antibodies and which over the years have proved to be very effective and an important innovation in the management of psoriasis. While the underlying cause of psoriasis remains to be determined, several lines of evidence point to the involvement of various interleukins as important disease drivers. Research has identified how the interleukin-17 (IL-17) pathway which includes six similar agents, IL-17A – IL-17F plays a major role with two members of the IL-17 family, iIL-17A and IL-17F implicated in autoimmunity. In fact, IL17F appears to regulate pro-inflammatory gene expression and requires the IL-17A receptor, suggesting that both interleukins are involved in the pathology of psoriasis. Bimekizumab is the first monoclonal antibody which selectively inhibits both IL-17A and IL-17F and is therefore a potentially important advance in the management of patients with moderate to severe psoriasis.

Clinical efficacy
The EU approval of bimekizumab was supported by the results of three phase 3 clinical trials, all undertaken in patients with moderate to severe psoriasis. The first, BE READY, randomised 435 patients (4:1) to bimekizumab 320 mg every 4 weeks or placebo. The co-primary endpoint was a PASI90 (i.e., 90% improvement in disease severity compared to baseline) and the proportion of patients achieving a score of 0 (i.e., clear skin) or 1 (almost clear), based on a 5-point investigator global assessment (IGA) score after 16 weeks of treatment. The results showed that a staggering 91% of those assigned to bimekizumab achieved a PASI90 compared to only 1% in the placebo group. In the BE VIVID trial, 567 patients were randomised to bimekizumab (at the same dosage as the BE READY trial) or this time, ustekinumab 45 or 90 mg every 12 weeks which is an active comparator, or placebo. Once again at week 16 there was a high response in the bimekizumab group with 85% achieving a PASI90 compared to 50% in the ustekinumab. The third trial, BE SURE, enrolled 478 patients who were randomised to either bimekizumab (same dosage) or adalimumab (another active comparator) at a dose of 40 mg every 2 weeks. At week 16, a PASI90 was achieved 85.3% of those using bimekizumab and 57.2% of those given adalimumab.
The most common adverse effects from bimekizumab are upper respiratory tract infections (14.5%) and patients are advised to seek medical advice if they display symptoms suggestive of an infection.
The EU approval applies to all 27 member states as well as Iceland, Liechtenstein and Norway at a dose of 320 mg administered by subcutaneous injections every 4 weeks for week 16 and every 8 weeks thereafter. The drug is currently under review by the US Food and Drug administration.
Source: UCB press release 2021