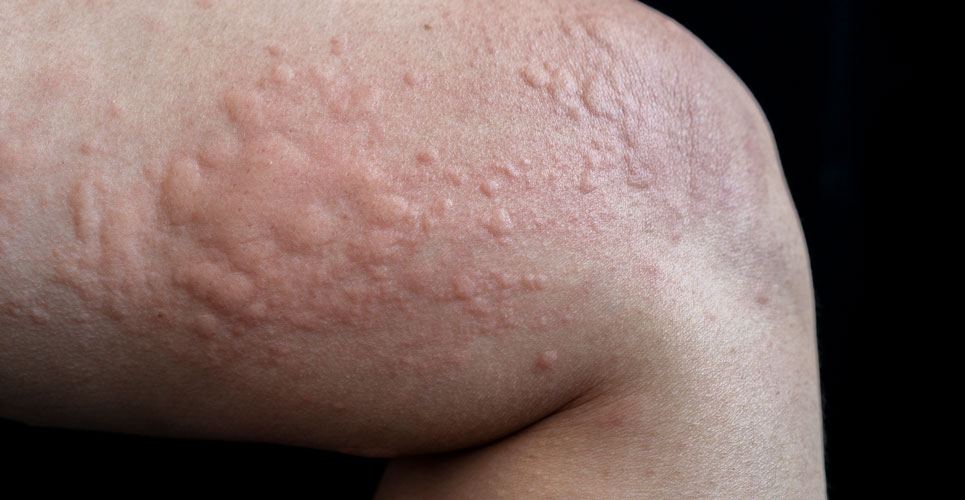
Lirentelimab is an anti-sialic acid binding immunoglobulin-like lectin 8 which appears to be effective for patients with chronic urticaria
Lirentelimab use in patients with chronic urticaria improves disease control in patients when assessed in terms of an increase in urticaria control test scores. This was the finding of a study by a team from the Dermatological Allergology, Allergie-Centrum-Charite, Department of Dermatology and Allergy, University of Berlin, Berlin, Germany.
Urticaria can be described as a superficial swelling of the skin leading to a red, raised and intensely pruritic rash and recurrent episodes occurring at least twice a week for 6 weeks are termed ‘chronic urticaria’. The global prevalence of chronic urticaria shows considerable variation, with a 2020 systematic review finding a higher point prevalence in Asian (1.4%) compared to European (0.5%) and Northern American (0.1%) studies. The primary effector cell in chronic urticaria is the mast cell which are located in the upper dermis and increased numbers are found in both lesional and non-lesional skin urticarial skin. The aim of treatment in chronic urticaria is achievement of complete control of symptoms and oral non-sedating antihistamines are the first-line recommendation. However, at licensed doses, anti-histamines provide effective symptomatic relief in less than 50% of patients. Although guideline-recommended up-dosing up to fourfold increases symptom control for some patients, many derive little benefit from antihistamines, highlighting the need for alternative therapies. Other effective treatments for chronic urticaria include the monoclonal antibody, omalizumab, which has be shown to be effective for those resistant to antihistamine therapy.
Sialic acid-binding immunoglobulin-like lectin-8 (Siglec-8) are cell surface proteins found predominantly on cells of the immune system. Siglec-8 is uniquely expressed by human eosinophils and mast cells and binding of monoclonal antibodies such as lirentelimab to Siglec-8 has been shown to inhibit IgE-mediated mast cell activation.
Lirentelimab has been shown to prevent passive systemic anaphylaxis through mast cell inhibition and given this finding, for the present, open-label, phase 2 study, the German researchers sought to evaluate the impact of lirentelimab on symptom control in patients with chronic urticaria.
They recruited individuals with a diagnosis of chronic urticaria for 3 or more months that was uncontrolled based on an urticaria control test score (UCT) of less than 12. Patients received up to six intravenous infusion doses of lirentelimab, ranging from an initial 0.3 mg/kg up to 3 mg/kg if tolerated. The primary efficacy outcome was the change in UCT scores from baseline to week 22 and a secondary outcome the proportion of those achieving a complete response (CR). Enrolled patients were categorised based on their chronic urticaria subtype as omalizumab-naïve, omalizumab-refractory, chronic spontaneous urticaria and chronic inducible urticaria.
Lirentelimab and UCT scores
A total of 45 patients with a median age of 42 years (74% female) were enrolled in the study. Patients in each of the subgroups experienced an improvement in UCT scores. Mean increases from baseline were 11.1 for omalizumab-naive, 4.8 for omalizumab-refractory, 6.5 for chronic spontaneous urticaria and 3.4 for chronic inducible urticaria.
For the secondary outcome, 92% of omalizumab-naive, 36% of omalizumab-refractory and 82% of those with chronic spontaneous urticaria, achieved a complete response at week 22.
The authors concluded that lirentelimab showed efficacy across a range of chronic urticaria subtypes including those refractory to omalizumab.
Citation
Altrichter S et al. An open-label, proof-of-concept study of lirentelimab for antihistamine-resistant chronic spontaneous and inducible urticaria J Allergy Clin immunol 2022