Rainer Oberbauer
MD
Department of Internal Medicine Division of Nephrology University of Vienna, Austria
E:[email protected]
Renal transplantation is the treatment of choice for endstage renal disease. Since the clinical introduction of the immunosuppressant cyclosporin A (CSA) in 1983, the one-year graft survival rate has continuously improved. More than 90% of all renal transplant recipients now have a functioning graft at one year after transplantation.(1)
However, this success in short-term allograft survival has not projected into long-term graft survival. The graft half-life is reported to have increased from 8 years to 13 years for cadaveric donor kidneys over the last decade.(2) But other reports from a large US database found only a moderate increase over the last five years, and a prolonged projected graft half-life of 8.5 years.(1)
The most common cause of graft failure with a functioning transplant, besides death, is chronic allograft nephropathy. This term incorporates many features of graft pathology. Clearly there are alloantigen-dependent and independent mechanisms that contribute to this process. One of the most important alloantigen-independent mechanisms is drug nephrotoxicity. Because drugs that are commonly associated with nephropathy in nontransplant patients are not used in renal transplant recipients, immunosuppressant nephrotoxicity is a major contributor to chronic allograft nephropathy. This is especially seen with the calcineurin antagonists, such as CSA or tacrolimus, which have been shown to contribute to chronic transplant failure.(3,4) Both drugs are similar in function and toxicity.
Gold standard in immunosuppression of renal transplant recipients
So far, the gold standard immunosuppressive regimen for renal allograft recipients consists of triple therapy with corticosteroids, an antiproliferative agent such as azathioprine or mycophenolate mofetil, and a calcineurin- antagonist. With this regimen an average graft half-life of 10 years could be achieved.(5) It was noted as early as one year after its clinical introduction in 1983 that CSA is nephrotoxic. Myers et al. from Stanford University reported 17 patients who received azathioprine immunosuppression after heart transplantation and 15 patients who received CSA. The latter had significantly lower glomerular filtration rates at one year after transplantation, compared with the azathioprine group.(6)
Recently there have been very promising clinical data for noncalcineurin antagonist immunosuppressants, which will change the current practice and philosophy of immunosuppression. This is discussed later in this article.
Functional and morphological acute and chronic toxicity
Functional changes
Calcineurin antagonists have been shown to cause acute and chronic nephrotoxicity. The acute depression of glomerular filtration is especially deleterious in the engraftment phase of renal transplantation. Postischaemic acute renal failure develops in about one quarter of all cadaveric donor kidneys. Although virtually all patients recover from postischaemic injury and renal allograft function is initiated, postischaemic renal transplant failure is one of the major risk factors for the development of chronic allograft nephropathy. CSA has been shown to increase the duration until recovery, when compared with other induction protocols without calcineurin antagonists.(7) The acute depression of glomerular filtration is due to renal vasoconstriction and the resulting reduction of renal blood flow.(8) CSA acts as a potent vasoconstrictor, also evident from the fact that CSA causes systemic arterial hypertension in patients without renal disease.(6) Post-transplant arterial hypertension is one of the major risk factors and thereby predictors of long-term graft survival, as has been shown very elegantly by Opelz et al.(9) Renal transplant recipients without hypertension at one year after engraftment had a much longer graft half-life compared with patients with hypertension. The graft half-life correlated with the degree of hypertension at one year after transplantation.(9)
Besides vascular changes, calcineurin antagonists cause renal tubular dysfunction, as evidenced by the impairment to adequate excretion of uric acid, hydrogen anions or potassium. The consequences are hyperuricaemia, distal tubular acidosis and hyperkalaemia. On the other hand, there is impaired reabsorption of phosphate in the proximal tubule and magnesium in the thick ascending limb of the loop of Henle. The phosphate leak is even more prominent under TOR (target of rapamycin)-inhibition.(10) The clinical consequences are hypophosphataemia and hypomagnesaemia.
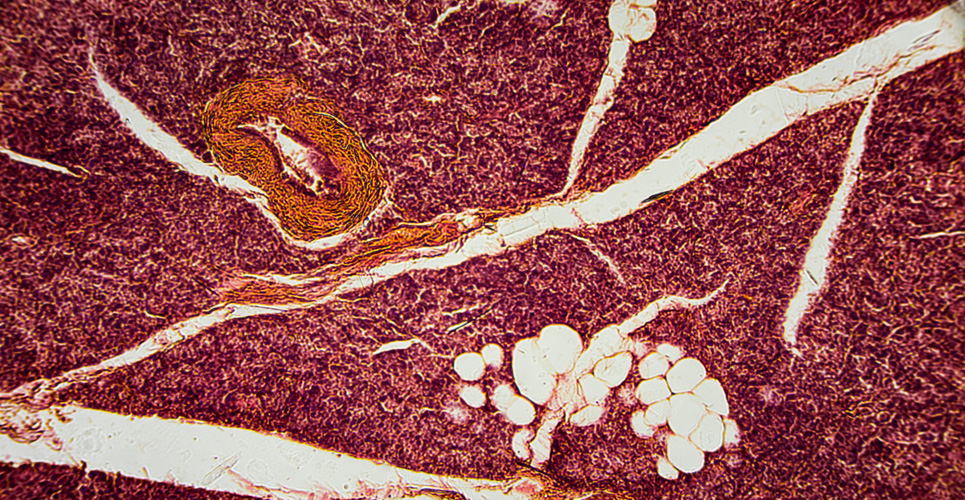
Morphological changes
Chronic nephrotoxicity of calcineurin antagonists cannot be diagnosed by distinctive, specific histomorphological criteria, but rather can be suggested if clinical and some of the following morphological features are present: striped interstitial fibrosis, tubular atrophy, and glomerulopathy with double contours of the basement membrane. Apoptosis of tubule epithelial cells has been reported for virtually all immunosuppressant drugs.(11–13) One study investigated renal biopsy specimens from 192 patients who had received CSA for between 6 and 40 months for nonrenal indications, such as autoimmune uveitis, new onset of type 1 diabetes or rheumatic disease.(14) Results showed that the degree of nephrotoxicity was proportional to the dose per body weight. Below 5mg/kg per day, no morphological features suggestive of CSA nephrotoxicity could be found. Between 7.5 and 15mg/kg per day, about 27% of patients exhibited typical findings of CSA toxicity in the renal biopsies. Above 15mg/kg per day, CSA nephrotoxicity could be diagnosed in 40% of patients.
Renal transplant recipients with long-term CSA therapy were morphometrically analysed by Pagtalunan et al.(15) The most striking morphological finding in the serially sectioned biopsy cores was that 18% of all unsclerosed, intact glomeruli had no connection to a tubule. The volume fraction of tubule epithelial cells was only 36% in these biopsies, but 59% and 71% respectively in cadaveric and living donor kidney biopsies obtained before transplantation. The continuous replacement of functional tubule epithelial cells by fibroblasts might be related to the maintenance therapy with calcineurin inhibitors.
Future perspectives
Until recently there have been no good alternatives to calcineurin antagonists for maintenance immunosuppression of solid organ transplant recipients. Now a new, non- nephrotoxic drug for maintenance immunosuppression is available. Rapamycin has potent antiproliferative effects – it inhibits the cell cycle progression from G1 to the S-phase by blocking mTOR (mammalian target of rapamycin) (see box below). TOR-downstream signals, such as the 40S ribosomal protein S6 kinase (p70s6k), and the function of the eukaryotic initiation factor 4E-binding protein-1 (4E-BP1), as well as cyclin-dependent kinases are inhibited.
This substance has no effect on the glomerular filtration rate, even when huge doses are used.(8) In clinical studies, rapamycin has proven to be safe and efficient.(18,19) The withdrawal of nephrotoxic immunosuppressants such as CSA from a combination with rapamycin and steroids can be performed safely. This CSA elimination is associated with less hypertension, the key risk factor for chronic transplant failure, and better excretory allograft function.
An individually designed maintenance immunosuppressive protocol based on protocol biopsy findings will be the future in the field of transplant immunosuppression. Combination therapy consisting of calcineurin antagonists and rapamycin will be used in the first months after transplantation. When the graft function is stable and the risk of acute rejection is low, a protocol biopsy will be used to guide a rational maintenance immunosuppressive therapy. This will consist of rapamycin in combination with a low dose of a calcineurin antagonist in the majority of patients. Patients who still exhibit signs of CSA nephrotoxicity can be treated with rapamycin monotherapy and low-dose steroids.
Conclusion
Renal transplant nephropathy is the main cause of chronic allograft failure. Immunosuppressant nephrotoxicity is among the most important alloantigen-independent contributors to this process. No alternatives to calcineurin antagonist maintenance immunosuppression have been available until recently. Rapamycin has been introduced into transplantation, and the first controlled clinical phase III studies are being conducted and show very promising results with regards to efficacy without nephrotoxicity.
References
- USRDS. Annual data report. 2001 Available from URL http://www.usrds.org.
- Hariharan S, Johnson CP, Bresnahan BA, et al. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med 2000; 342:605-12.
- Johnson RW. The clinical impact of nephrotoxicity in renal transplantation. Transplantation 2000;69:SS14-7.
- Davies DR, Bittmann I, Pardo J. Histopathology of calcineurin inhibitor-induced nephrotoxicity. Transplantation 2000;69:SS11-3.
- Cecka JM. The UNOS Scientific Renal Transplant Registry 2000. Clin Transpl 2000:1-18.
- Myers BD, Ross J, Newton L, et al. Cyclosporine-associated chronic nephropathy. N Engl J Med 1984; 311:699-705.
- Canafax DM, Torres A, Fryd DS, et al. The effects of delayed function on recipients of cadaver renal allografts. A study of 158 patients randomized to cyclosporine or ALG-azathioprine. Transplantation 1986;41:177-81.
- Sabbatini M, Sansone G, Uccello F, et al. Acute effects of rapamycin on glomerular dynamics: a micropuncture study in the rat. Transplantation2000;69:1946-90.
- Opelz G, Wujciak T, Ritz E. Association of chronic kidney graft failure with recipient blood pressure. Collaborative Transplant Study. Kidney Int 1998;53:217-22.
- Schwarz C, Bohmig GA, Steininger R, et al. Impaired phosphate handling of renal allografts is aggravated under rapamycin-based immunosuppression. Nephrol Dial Transplant 2001;16:378-82.
- Peherstorfer E, Mayer B, Böhm S, et al. Effects of microinjection of synthetic Bcl-2 family member domain peptides on apoptosis of renal proximal tubular epithelial cells in vivo (personal communication; 2001).
- Shihab FS, Andoh TF, Tanner AM, et al. Expression of apoptosis regulatory genes in chronic cyclosporine nephrotoxicity favors apoptosis. Kidney Int 1999;56:2147-59.
- Longoni B, Boschi E, Demontis GC, et al. Apoptosis and adaptive responses to oxidative stress in human endothelial cells exposed to cyclosporin A correlate with BCL-2 expression levels. Faseb J 2001;15:731- 40.
- Feutren G, Mihatsch MJ. Risk factors for cyclosporine-induced nephropathy in patients with auto-immune diseases. International Kidney Biopsy Registry of Cyclosporine in Autoimmune Diseases. N Engl J Med 1992;326:1654-60.
- Pagtalunan ME, Oberbauer R, Haas M, et al. Atubular glomeruli in patients with chronic allograft rejection. Transplantation 1996;61:1166-71.
- Sehgal SN, Baker H, Vezina C. Rapamycin (AY-22,989), a new antifungal antibiotic. II. Fermentation, isolation and characterization. J Antibiot (Tokyo) 1975;28: 727-32.
- Vezina C, Kudelski A, Sehgal SN. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle.J Antibiot (Tokyo) 1975;28:721-6.
- Kahan BD. Efficacy of sirolimus compared with azathioprine for reduction of acute renal allograft rejection: a randomised multicentre study. The Rapamune US Study Group. Lancet 2000;356:194-202.
- MacDonald AS. A worldwide, phase III, randomized, controlled, safety and efficacy study of a sirolimus/cyclosporine regimen for prevention of acute rejection in recipients of primary mismatched renal allografts. Transplantation 2001;71:271-80.
Resources
European Renal Association – European Dialysis and Transplant Association
W:www.era-edta.org/erafset.htm
European Society
of Organ Transplantation
W:www.esot.org
Nephronline
W:www.nephronline.org
Forthcoming events
14–17 July 2002
XXXIX Congress
ERA–EDTA
Copenhagen Denmark
W:www.unipr.it/~eraedta/2002.htm
20–25 Sept 2003
11th Congress of the ESOT
Venice, Italy
Local Organising Committee:
Ermanno Ancona
T:+39 49 8211240 /8211720
F:+39 49 750919
E:[email protected]