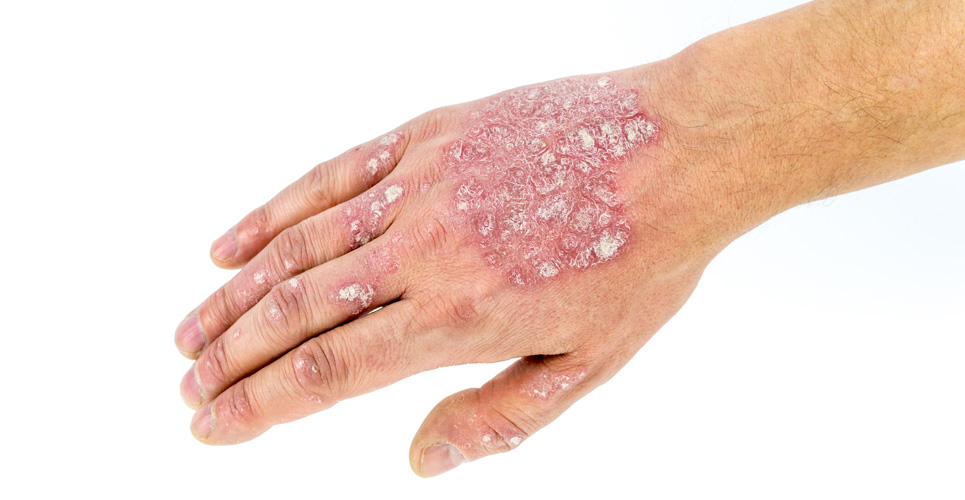
The Scottish Medicines Consortium (SMC) has accepted Enstilar® (calcipotriol/betamethasone dipropionate) for use within NHS Scotland.1
This decision confirms that GPs and dermatology clinicians are now authorised to prescribe Enstilar® for the management of all severities of plaque psoriasis in adult patients, aged 18 years or older.2 The SMC’s positive recommendation for Enstilar® follows its UK Marketing Authorisation on 15 April 2016.
The Scottish Medicines Consortium (SMC) has accepted Enstilar® (calcipotriol/betamethasone dipropionate) for use within NHS Scotland.1

This decision confirms that GPs and dermatology clinicians are now authorised to prescribe Enstilar® for the management of all severities of plaque psoriasis in adult patients, aged 18 years or older.2 The SMC’s positive recommendation for Enstilar® follows its UK Marketing Authorisation on 15 April 2016.
The National Institute for Health and Care Excellence (NICE) does not need to review the calcipotriol/betamethasone dipropionate cutaneous foam spray as the combination treatment does not meet with their criteria for Health Technology Appraisals (HTAs) as these two active agents are well established in the NHS and the market.
Following an abbreviated submission, the SMC’s decision states that: “calcipotriol and betamethasone cutaneous foam (Enstilar®) is accepted for use within NHS Scotland, indicated for the topical treatment of psoriasis vulgaris in adults.” The SMC also advises that “Enstilar® cutaneous foam is another licensed formulation of calcipotriol/betamethasone and may be associated with a small ”budget impact”.1
Clinical studies show that this calcipotriol/betamethasone dipropionate foam spray is a more effective topical combination treatment than those currently available and is generally well-tolerated.3,4 More than half of patients in clinical trials experienced significant visible signs of improvement within four weeks, with some patients seeing improvements at one week.5
References
- Scottish Medicines Consortium: calcipotriol 50 micrograms/g and betamethasone 0.5g cutaneous foam (Enstilar®). SMC. Number 1182/16. Published Monday 12th September, 2016. Available at: www.scottishmedicines.org.uk
- Enstilar® SmPC; UK. Available at: http://www.medicines.org.uk/emc/medicine/31833. Last Accessed August 2016
- Koo J et al. Superior efficacy of calcipotriene and betamethasone dipropionate aerosol foam versus ointment in patients with psoriasis vulgaris – A randomized phase II study. J Dermatol Treat 2015; 1471-1753
- Taraska V et al. A novel aerosol foam formulation of calcipotriol and betamethasone has no impact on HPA axis and calcium homeostasis in patients with extensive psoriasis vulgaris. J Cutaneous Med Surg 2015; 1-8
- Leonardi C et al. Efficacy and safety of calcipotriene plus betamethasone dipropionate aerosol foam in patients with psoriasis vulgaris – a randomized phase III study (PSO-FAST). J Drugs Dermatol 2015; 14(12): 1468-77