teaser
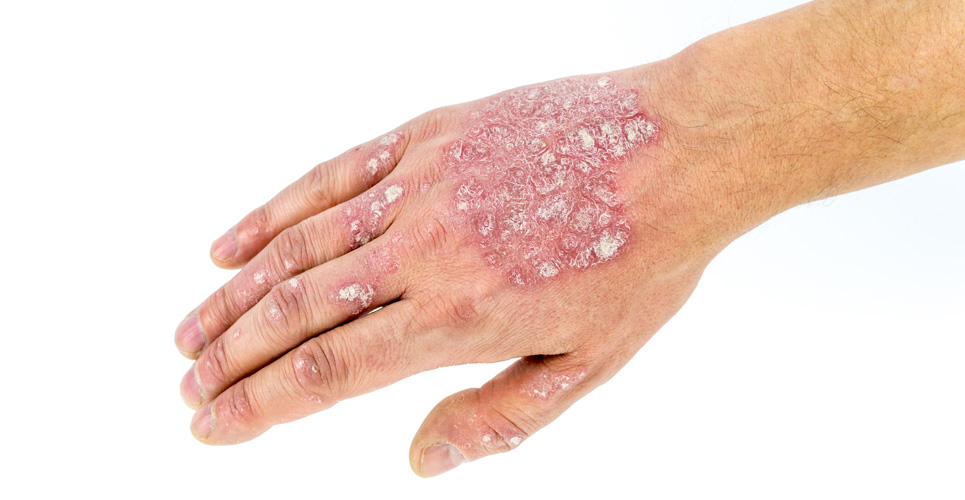
Psoriasis patients experience a significant reduction in the signs of their disease when treated with adalimumab (Humira).
Data from the phase III REVEAL (Randomized Controlled EValuation of Adalimumab Every Other Week Dosing in Moderate to Severe Psoriasis TriAL) study evaluated both short-term and sustained safety and efficacy of adalimumab in 1,200 patients with moderate to severe chronic plaque psoriasis. The two primary endpoints were of the proportion of patients achieving 75% improvement in skin clearance after 16 weeks, and the proportion of patients who lost adequate response through week 52 after stopping treatment at week 33. Patients were randomised to receive either adalimumab (40mg every other week beginning at week 1 after a starting dose of 80mg at week 0) or placebo. Results showed that 71% of patients achieved at least 75% improvement in disease signs (PASI 75) after 16 weeks of adalimumab, compared with 6.5% of patients who achieved PASI 75 after receiving placebo.
In the second primary endpoint measured at week 52, 28% of patients receiving placebo lost adequate response, compared with 5% of patients receiving adalimumab (loss of adequate response was defined as less than 50% improvement at any time between week 33 and 52, compared with the beginning of the study and as worsening extent and severity of disease from week 33 to week 52, as measured by a six-point increase in the PASI score).