The aim of this audit was to ensure the clinical pharmacists were accurately preparing the medication section of the Immediate Discharge Summary and providing the necessary information required by the GP detailed in the GAIN guidelines
The aim of this audit was to ensure the clinical pharmacists were accurately preparing the medication section of the Immediate Discharge Summary and providing the necessary information required by the GP detailed in the GAIN guidelines
Maire McManus MSc
Dianne Gill MSc
Allison Loomie BSc
Glenda Fleming PhD
Michael Scott PhD
Medicines Optimisation Innovation Centre*
Northern Health and Social Care Trust (NHSCT)
Email: [email protected]
*Formerly Pharmacy and Medicines Management Centre
The Immediate Discharge Summary (IDS) is one of the most crucial pieces of documentation in the health record, as it is the basis of communication between secondary and primary care and essential for ensuring quality and continuity of care. A GP needs access to this useful and potentially life-saving information quickly and it is critical to their ability to continue care uninterrupted when they resume responsibility for a patient’s care. A major issue with discharge documentation has been the provision of accurate medication information.1
The GAIN guidelines on regional immediate discharge documentation for patients being discharged from secondary into primary care,1 published in 2011, provided recommendations on the information GPs required on the IDS so they could safely manage their patients post-discharge.
In the study hospital, clinical pharmacists, Band 7 and above, are authorised by the Trust Protocol2 to prepare the medication section of the IDS following successful completion of an accreditation process.
The pharmacist preparing the medication section of the IDS, performs a clinical check, no further clinical check is obtained. A transcription check of the medication section of the IDS is carried out by pharmacy staff, either a pharmacist or an authorised pharmacy technician. Bespoke medicines reconciliation software (Writemed) has been developed through collaboration between the Pharmacy Department, IT Department and a commercial IT Company (Yarra software). Writemed is used by pharmacists to generate the medication section of the IDS, and this ensures that most of the information required by the GP, as detailed in the GAIN guidelines, is available in a legible electronic format. Clinical pharmacists complete admission medicines reconciliation electronically on Writemed, which contains a DM&D drug dictionary. When the clinical pharmacist completes the medicines reconciliation on discharge, using Writemed, change to the admission medication and allergy status are mandatory fields so must be completed. A discharge medication report is produced, which is incorporated into the IDS, which also contains the clinical narrative produced electronically by the junior doctor.
Aim
The aim of this audit was to ensure that the clinical pharmacists were accurately preparing the medication section of the IDS according to the Trust protocol2 and providing the necessary information required by the GP as detailed in the 2011 GAIN guidelines.1
Method
A random selection of 60 IDSs, which had the medication section completed by clinical pharmacists were reviewed by a senior clinical pharmacist along with the medical notes and computer laboratory test results. The accurate transcription of all medicines from the inpatient medicine kardex was confirmed and a clinical check was carried out on the IDS using information from the medical notes, laboratory results and admission medicines reconciliation form.
An audit data collection form was completed for each IDS. The pharmacists were unaware of the audit taking place and so no bias was expected. There were 16 clinical pharmacists writing the medication section of the IDS on 10 medical wards in the study hospital. Seven of the sixteen pharmacists audited were non-medical prescribers. The data collection period was over two weeks in June 2014.
A validation check was performed by a senior clinical pharmacist from another hospital in the Trust. A list of 14 patients who had the medication section of the IDS prepared by a range of pharmacists were provided. The random sample included some with errors and some with no errors. This pharmacist completed the same audit data collection form for the 14 patients and graded any errors found using the Eadon intervention grading scale.3
Results
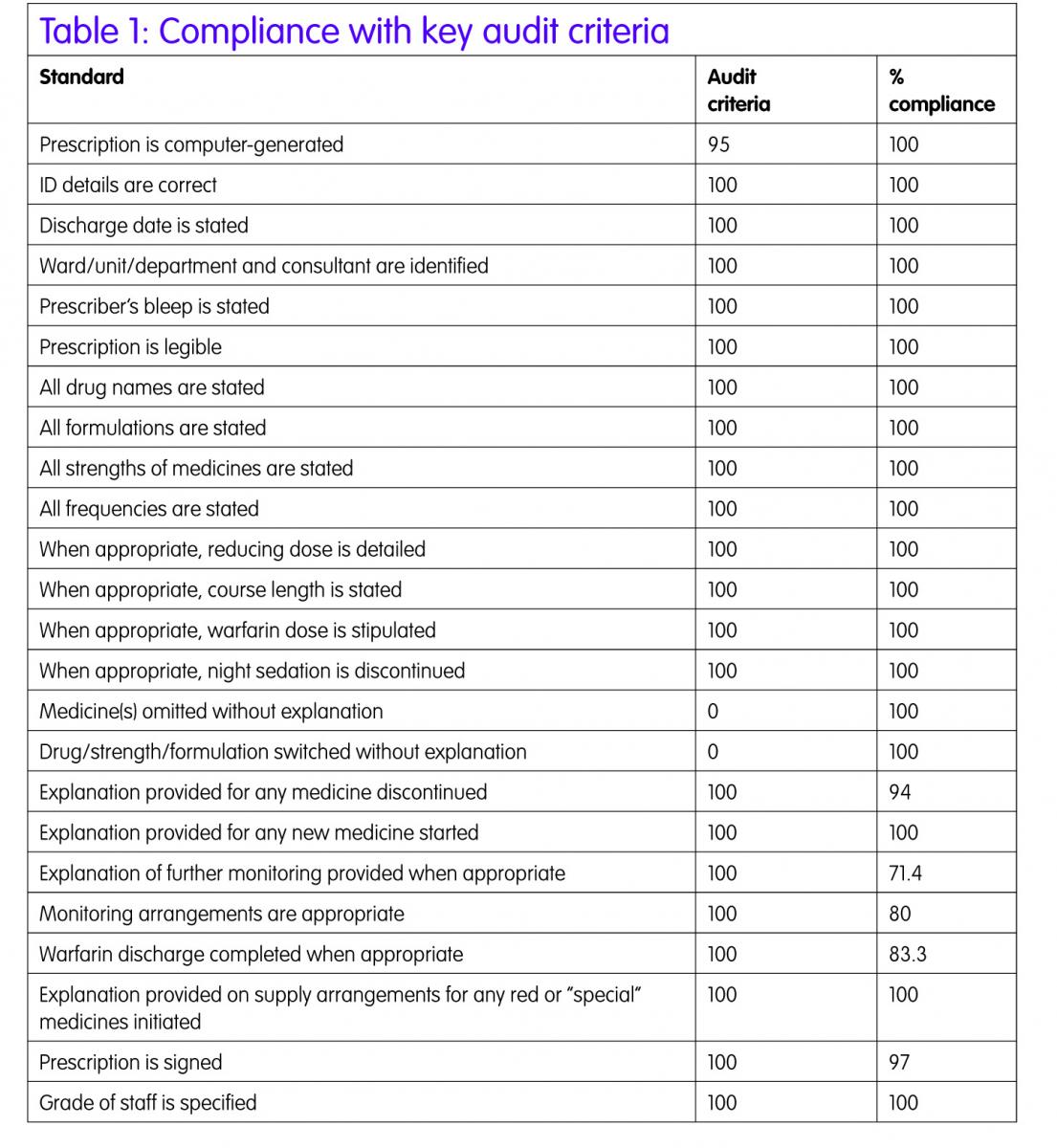
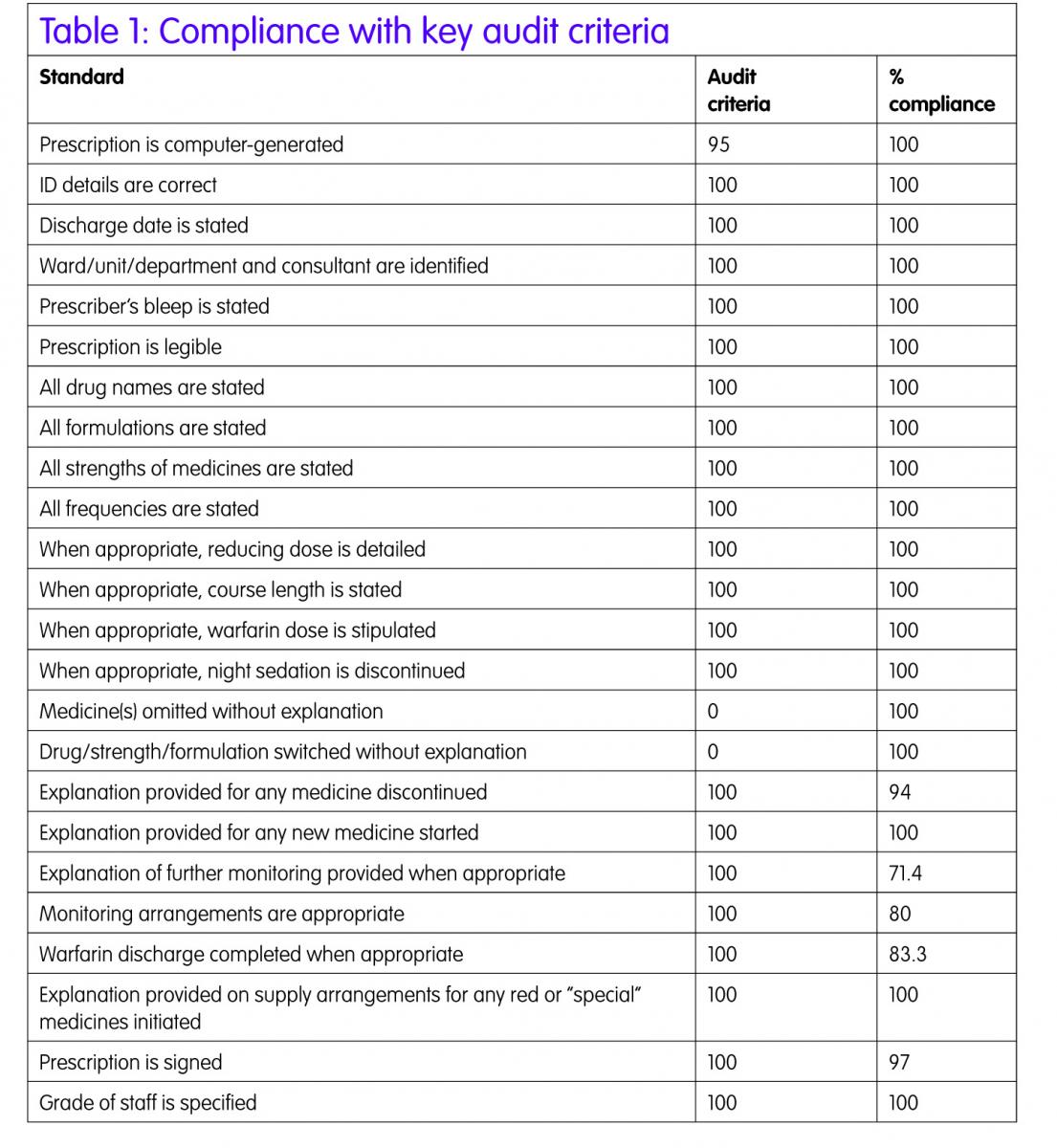
The majority of the 41 standards audited were met (Table 1). The first part of the audit considered the general information and legal requirement standards, a high level of compliance was achieved. Allergy status was correctly stated in 100% of occasions. The Writemed software ensures that this information is recorded on the medication section of the IDS as the allergy status is a mandatory field to be completed before the discharge report can be finalised. The other information automatically populates the discharge report, as this information is exported from the Patient Administration System (PAS) into Writemed.
The second part of the audit considered clinically relevant issues, as detailed in the Trust protocol2 to ensure the pharmacists were compliant with the protocol.2
Medicines reconciliation was completed on discharge for all patients using the Writemed system. This ensures that any medication changes made during the hospital stay are clearly documented on the discharge report with reasons for changes. This ensures that the information about dose changes, medicines stopped, medicines held during admission and then restarted on discharge and new medicines commenced with reasons are documented on the medication section of the IDS. Having the drug dictionary incorporated into the Writemed software reduces the risk of incorrect spelling of medication which is frequently seen in the medication section of the IDS prepared by junior doctors where the medicines are free-typed.
The reducing course and stop date for courses of medication were clearly stated on the IDS in all cases.
Where warfarin was prescribed (n=6), a warfarin discharge letter was prepared for the majority of patients (5/6) detailing previous INRs and doses administered, future doses and date of next INR check.

One area that was difficult to audit was drug interactions, drug–disease incompatibilities and duplication of therapy. In 20% of the IDS audited, drug interactions as specified in the BNF were found but many patients had been stable on these drug combinations prior to admission and it was not thought to be a contributing factor for the admission.
The same issue applied for drug–disease incompatibilities (11.6%), in all cases the patients were prescribed the incompatible medicine prior to admission. The pharmacist may have discussed this with the medical staff when completing medicines reconciliation on admission or discharge but this was not documented in the patient’s notes and so could not be determined for the audit.
Duplication of therapy (6.67%) can occur for good therapeutic reasons, for example co-prescription of two anti-hypertensive medicines, but there were a small number of cases, which both pharmacists completing the audit form judged to be erroneous duplications. The pharmacist may have raised the duplication issue with the medical team during the hospital stay but again this was not documented in the patients’ notes.
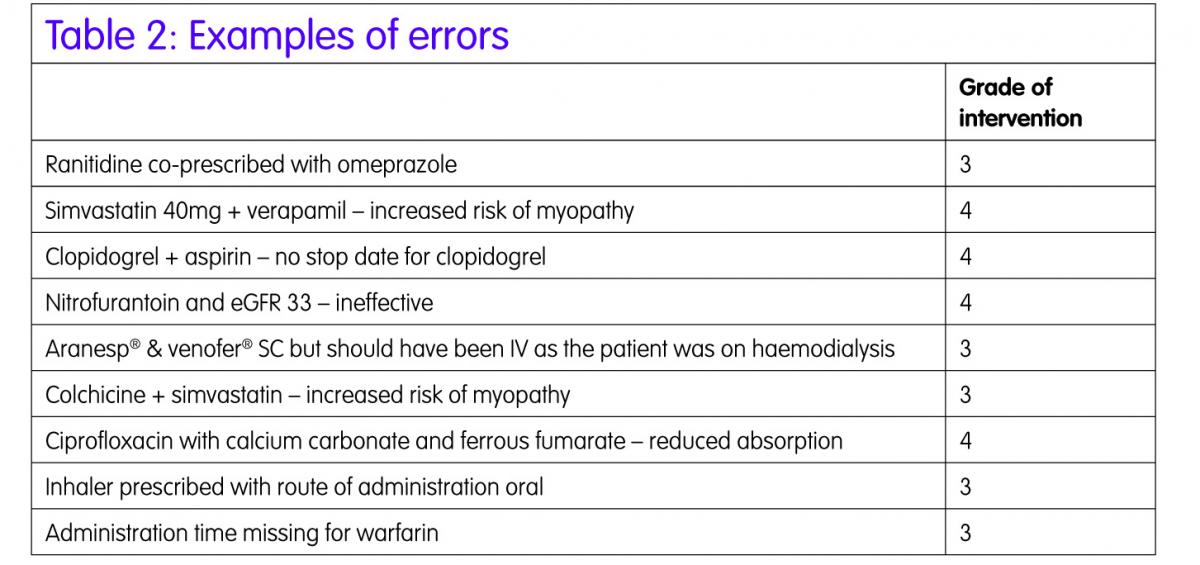
In the audit a 1.3% error rate per prescribed item was found. Table 2 gives an example of some of the errors.
These fell into three main categories drug interactions, drug–disease incompatibilities and duplication of therapy. Some of the errors relate to medications that patients were prescribed prior to admission to hospital, so may have been discussed with the medical staff and deemed appropriate, but this was not documented in the notes by the pharmacist. This highlights the need for more sophisticated decision support systems, for example clinical rules,4 which will individualise prescribing advice depending on the patients’ laboratory results and other co-morbidities.
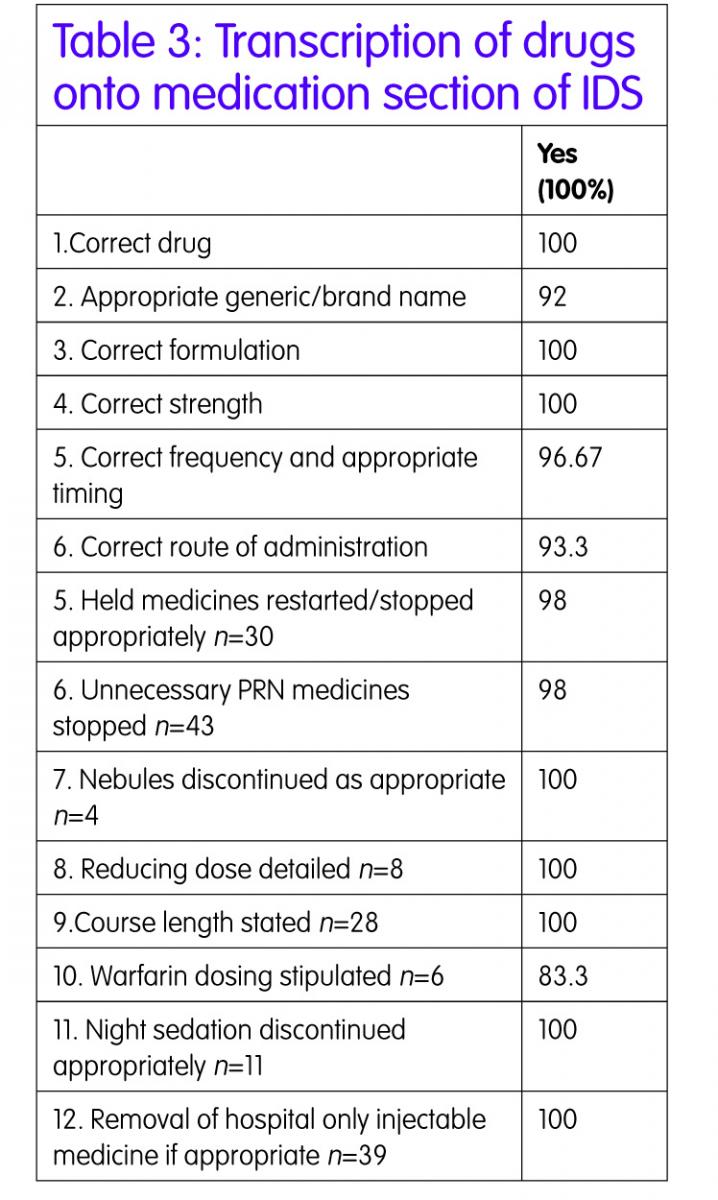
Transcription of medication from the medicine kardex to the IDS was 100% accurate in terms of drug name, formulation and strength. This would be expected as there is a transcription check built into the process and so transcription errors should be picked up and corrected before the patient is discharged. There were two minor frequency discrepancies for example metformin was not specified with breakfast. There were three minor errors with route of administration, for example nebules route was stated as ‘inhaled’ instead of ‘nebulised’.
All but one script had held medicines restarted/stopped appropriately. Nitrofurantoin was restarted as a prophylactic antibiotic when the patient’s eGFR was 37ml/min so this medicine would be ineffective and an alternative should have been sought. This may have been discussed with medical staff but there was no documentation of this in the patient’s notes.
New medicines and dose altered information was required for 83.3% of the IDS prepared and this information was appropriate in all cases. Information about medicines stopped was provided in almost all cases and sometimes the explanation was very comprehensive and other times it just said ‘stopped as per Dr’ because there was no apparent reason in the medical notes. Sometimes the reason given was inappropriate, for example ramipril was stopped and the reason stated was ‘low blood pressure’ but the patient also had AKI so perhaps both reasons needed to be recorded so the GP knows to monitor the BP and U&Es before restarting.
Monitoring requirements detailed were variable. More than half of the IDS prepared required the GP to monitor the patient post-discharge. This may have been to monitor blood pressure but the majority of cases it was to repeat blood tests. Examples of times when monitoring requirements were not specified were when patients had diuretics or ACE inhibitors restarted at discharge or dose increases during the inpatient stay and the GP should have been advised to monitor U&Es.
A validation check was completed by a second senior clinical pharmacist. Full agreement was reached in nine of the random selection of fourteen IDSs audited. There were professional variations in auditing drug interactions and monitoring requirements but the vast majority of errors recorded were of minor or moderate significance and were graded as 3 and grade 4.
Conclusion
The majority of audit standards were achieved. Clinical pharmacists are accurately preparing computer generated discharge medication information on the IDS, which contains accurate and comprehensive information. This greatly improves the quality of information supplied to GPs and ensures patients’ medicines are reconciled better across the secondary–primary care interface.
The audit has highlighted that improvement is required on documentation of decisions made following discussions with medical staff. There is also a need for more guidance to be given to clinical pharmacists on what are considered relevant laboratory results and what are relevant monitoring requirements to request the GPs to undertake.
Utilising the Writemed medicines reconciliation software has been key to improving the quality of discharge medication information because it ensures that all the information required by the GPs as detailed in the GAIN guidelines1 is recorded. The warfarin discharge information was added to the IDS template in March 2015, which ensures that warfarin dose information is readily available in electronic format, so pharmacy staff will not be reliant on paper copies.
All pharmacists are working within the Trust protocol to prepare the medication section of the IDS. Pharmacists preparing the medication section of the IDS ensure that medicines reconciliation is completed accurately at discharge and the GP receives the information required to safely follow-up their patients in primary care. The GAIN Guidelines on regional immediate discharge documentation for patients being discharged from secondary into primary care1 were published in June 2011 and contained essential information required by GPs on the discharge letter. The Writemed and IDS format utilised within the process ensures compliance with GAIN guidelines by providing the information required by GPs when the patient is discharged back into primary care.
The results of this audit were presented to the clinical pharmacists at the monthly team meeting to highlight areas of excellence and areas for improvement. The plan is to re-audit biennial to ensure we continue to improve the accuracy of the discharge medication provided to GPs and show improvement in the areas that were identified as needing further work.
Key points
- Pharmacist preparation of the medication section of the IDS ensure all medicines are reconciled at discharge.
- Pharmacist preparation of the medication section of the IDS ensure all medication related information is communicated to Primary Care.
- Writemed software has been key to improving the quality of the discharge medication information
- There is no requirement for a second clinical check by a pharmacist.
References
- Guidelines and Audit Implementation Network (GAIN) guidelines on regional immediate discharge documentation for patients being discharged from secondary into primary care, June 2011.
- Trust protocol for clinical pharmacist to prepare and authorise discharge medication records for general practitioners, updated April 2015.
- Eadon H. Assessing the quality of ward pharmacists interventions. Int J Pharm Pract 1992;1(3):145–7.
- Van Roon EN et al. Clinical relevance of drug–drug interactions: a structured assessment procedure. Drug Safety 2005;28:1131–9.