teaser
An ointment to limit flare-ups of dermatitis between regular treatments with corticosteroids or calcineurin inhibitors has been cleared for use by the European Medicines Agency (EMEA).
Its Committee for Medicinal Products for Human Use (CHMP) has recommended that Astellas Pharma Europe`s Protopic (tacrolimus monohydrate) be approved for twice-weekly use.
The recommendation will now be considered for final EC approval, which is likely to take between two and three months.
Studies have found that a twice-weekly application of Protopic after regular treatments with the anti-inflamatory agents significantly reduces the number of flare-ups.
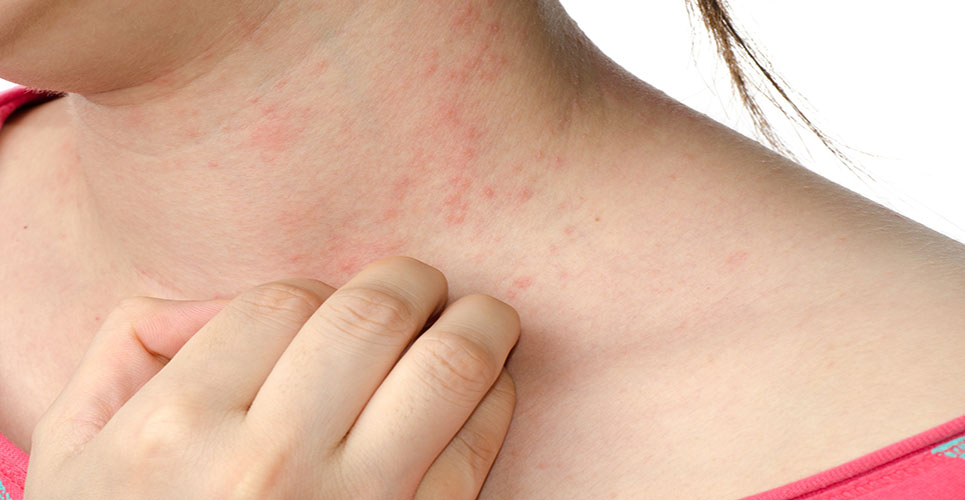
Atopic dermatitis, also known as atopic eczema, affects 14-24% with an itchy skin condition, and has a major impact on the quality of life of sufferers. The CHMP’s decision follows two phase III CONTROL studies with 524 adults and children.
Said Dr Sakari Reitamo, at the Hospital for Skin and Allergic Diseases, Helsinki University Central Hospital: “One of the major problems for patients is the impact of recurring eczema flares.
“This innovative treatment, which suppresses inflammatory activity between flares, offers a major breakthrough in the reduction of flares to improve the long-term control of atopic eczema.”
Copyright Press Association 2009
Committee for Medicinal Products for Human Use