teaser
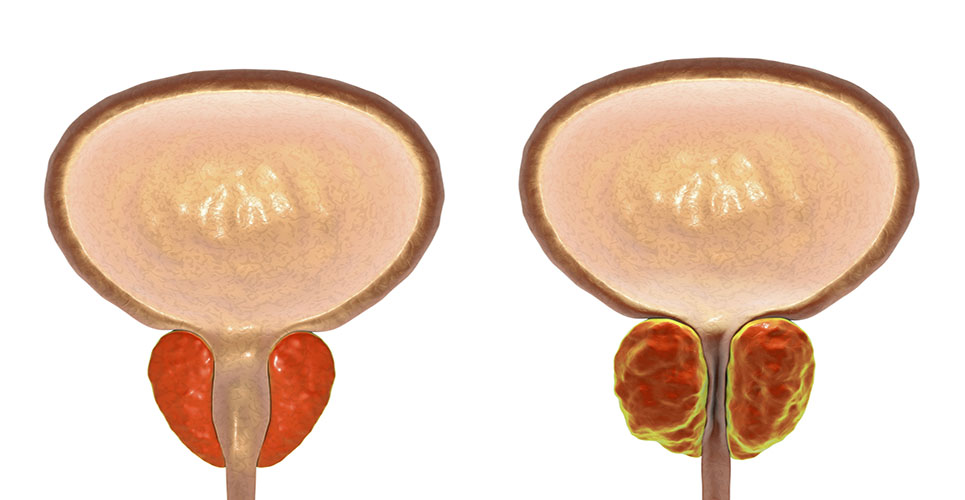
British drugmaker GlaxoSmithKline (GSK) is submitting approval updates for its prostate drug Avodart to European and US regulatory agencies.
The drug is already used to treat symptoms of an enlarged prostate, and the company is seeking approval for its use as a treatment to reduce the risk of prostate cancer.
Research has shown that Avodart reduces the risk of cancer by about 25%, and GSK says it wants to provide an update to its application to EU regulators and the US Food and Drug Administration (FDA).
The company says that its decision is not due to any new safety or effectiveness data. Thousands of pages of testing results are usually included in such applications.
Doctors reported in April that a large international study had found that Avodart, which is known chemically as dutasteride, reduces the likelihood of men being diagnosed with prostate cancer by 23% after four years’ of use.
Copyright © Press Association 2009