teaser
Basilea Pharmaceutica Ltd announces that Toctino (alitretinoin), a new once-daily oral treatment for adults with severe chronic hand eczema (CHE) unresponsive to potent topical corticosteroids, has been approved by the health authorities in Belgium (Agence Fédérale des Medicaments et des Produits de Santé [AFMPS]) and in Luxemburg (Ministère de la Santé [MS]).
Subsequent to the recommendation for regulatory approval under the European decentralised procedure, Basilea received the marketing authorisation for Toctino in Belgium and Luxemburg. Following the regulatory approval of Toctino in Belgium and Luxemburg, Basilea will submit a pricing and reimbursement dossier to the country authorities.
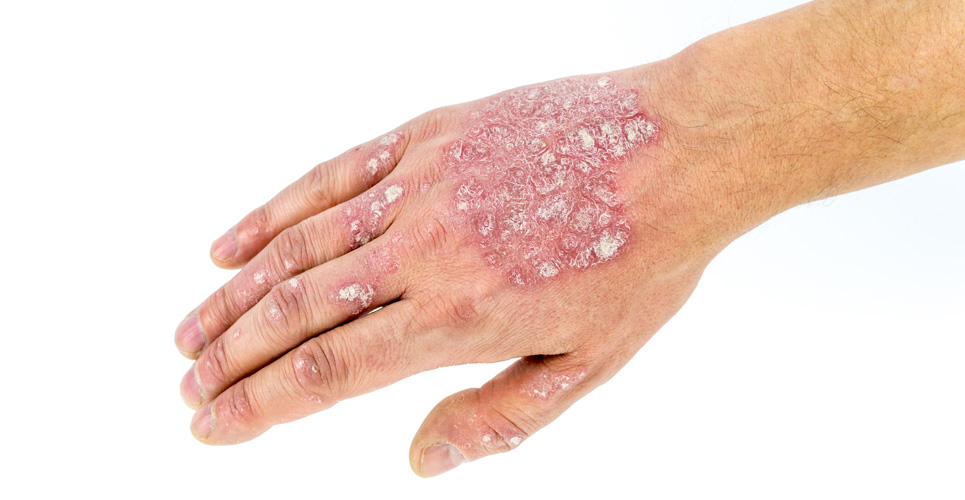
Chronic hand eczema – a debilitating skin disease
Hand eczema is a common inflammatory skin disease and is often chronic and relapsing. Hand eczema is reported to affect up to ten percent of the general population. The more severe,
chronic form of the condition is thought to affect five to seven percent of these patients, causing impaired use of their hands and a considerable impact on their ability to perform
everyday activities. Toctino, the only therapy approved for severe chronic hand eczema unresponsive to potent topical corticosteroids Toctino (alitretinoin) was developed by Basilea Pharmaceutica International Ltd.
The Belgian AFMPS and the MS in Luxemburg approved Toctino for the use in adults who have severe CHE that is unresponsive to treatment with potent topical corticosteroids.
Toctino is a once-daily oral therapy for the treatment of adults that is given for 12 to 24 weeks, depending on patient response. In the six-month post-treatment observation in the pivotal phase III clinical trials, patients who responded to Toctino experienced long periods free from relapse and improved patient satisfaction.
Toctino has been launched in Denmark, Germany and the United Kingdom and has also received marketing authorisation in Finland and France. It has also been recommended for approval in four additional EU Member States and is under regulatory review in Canada and Switzerland. In the largest ever phase III clinical trial program in CHE, Toctino was the first treatment able to show effective clearing of severe CHE unresponsive to potent topical corticosteroids, with clear or almost clear hands achieved in nearly 50 percent of patients treated with 30 mg Toctino.
Toctino is a known teratogen (a substance that can cause birth defects when women are exposed during pregnancy). Strict pregnancy prevention one month before, during, and one
month after cessation of treatment as well as monthly pregnancy testing are required for women of childbearing age.
A comprehensive pregnancy prevention program for Toctino has been developed and implemented. In clinical trials, Toctino was well tolerated and demonstrated a safety profile overall consistent with the retinoid class. Overall, the most frequently reported adverse events in the phase III clinical trials were headache and increased levels of blood lipids. Side effects were dose-dependent.
For more information please click on the link below: