There is a widespread public perception that prices for medicines are unfair, according to Suzannne Hill (Department of essential medicines and health products, World Health Organization (WHO)). “It is now important to consider how we should pay for new medicines, what we can afford, and how to make the system sustainable”, she continued.
There is a widespread public perception that prices for medicines are unfair, according to Suzannne Hill (Department of essential medicines and health products, World Health Organization (WHO)). “It is now important to consider how we should pay for new medicines, what we can afford, and how to make the system sustainable”, she continued.
The cost of medicines has risen steadily but the impact of medicines has not increased. Furthermore, manufacturers are receiving less money – in fact the return on investment for novel products is now negative, she explained. In addition, the products that are most needed, such as antibiotics and medicines for tropical diseases, are not being developed.
The main driver for medicines’ costs is always said to be cost of research and development (R&D) but, according to industry figures, R&D is only a small part of overall costs. Clinical trials, especially Phase III studies, are expensive in Europe because of regulatory requirements. Trials are now moving to cheaper environments but patients in these countries rarely get early access to the products. Nevertheless, despite agreements, more than half of all research is not published. This has been the case over the past ten years and it applies to all types of trials, regardless of site, sample size, study phase or funder.
Drug shortages are now ubiquitous and particularly affect injectable and small volume products. The price of generic drugs might have been pushed too low and a global strategy is now needed to support a minimum price that ensures quality and a market, said Dr Hill. The WHO has examined the problem and summarised the policy options.
Generic competition can be helpful but it is estimated that between seven and ten suppliers (of generic versions of a product) are needed to have effective competition and it is unlikely that there will be such large numbers of suppliers for some of the new molecules.
Reference-pricing might work but is fraught with difficulties. Managed entry agreements – outcome-based or non-outcome-based – might be the way forward, she suggested. An example of an outcome-based scheme is one in which payment is made for a medicine only when patients achieve a satisfactory outcome. Such schemes work provided that there a clear rules for prescribing and for measuring outcomes.
For some countries, affordability will always be a more important issue than value.
Summarising the position, Dr Hill said that we now need to reassess what we can afford to pay for cancer treatment, for antibiotics and for diseases of the poor. We need a fair and balanced approach to pricing of new products because without this we will not get innovation or “products on the shelf”. “It is important to realise that the models that we have relied on in the past are not continuing to deliver and we need new models such as public/private partnerships”, she said.
Big data – hype or help?
Systems-based medicine will enable Germany to cope with the growing population of people with Alzheimer’s disease and the shrinking numbers of people to care for them, said Heyo Kroemer (Dean of the Medical School, University of Göttingen, Germany). It is estimated that by 2050 there will be three million people in Germany with Alzheimer’s disease in a total population of 70 million (about 4%). As the birth rate has fallen in recent years, there will be a dwindling population to care for these people. Systems-based medicine uses computational, statistical and mathematical multiscale analysis and modelling of disease and treatment processes both at epidemiological and individual patient levels, he explained. It can only work if ‘big data’ are available – datasets so large or complex that traditional data processing applications are inadequate.
Professor Kroemer foresees that three types of big data will be relevant – conventional big data, unused big data and private big data.
Conventional big data are those which are derived from advanced analytics such as genomic analyses. The German cancer research centre is producing 11 terabytes (TB) of data each day – similar to Twitter which produces 12 TB a day, he noted.
Unused big data describes data generated in the process of routine clinical care but which are never used again. The Academic Medical Centre in Göttingen produces about 0.5 TB per day. These data could be coupled with medical records and used to identify genomic variants, rare diseases, and rare outcomes – much like the Vanderbilt DNA bank (BioVU) in the US. In Germany, patients with rare diseases are alone – it is not possible to identify others with similar conditions but unused big data could make this possible. In fact, phenome-wide association studies and genome-wide association studies could provide a tool for detection of rare diseases, if large databases are available, he said.
Private big data refers to the data stored in private devices such as smart phones – of which there are 45 million in Germany. This could be as simple as step monitors or more complex such as results from implantable glucose-monitoring chips or cardiovascular monitoring. Blood pressure monitoring and detection of atrial fibrillation will soon be possible on smart phones, he commented.
Ideally, in future it will be possible to integrate the three types of big data to enable true ‘systems medicine’ and more effective individual care.
There are some limitations at present. The biggest obstacle to progress in Germany is that the various systems cannot talk to each other. There are also non-medical questions that have to be answered, such as security of data and privacy issues.
Big data will affect all our jobs and we need to be engaged with it. We are living in a new age of enlightenment – the generation and assessment of data is increasingly autonomous – man is no longer the driving force, said Professor Kroemer. He concluded that the use of big data presents major opportunities but that they must be used responsibly.
Anticoagulation evidence
There is an “emerging epidemic” of atrial fibrillation (AF) in Europe according to Helen Williams (Consultant Pharmacist for Cardiovascular Disease, South London and Clinical Lead for Atrial Fibrillation, Health Innovation Network). AF is associated with increased risk of ischaemic stroke and there are approximately 360,000 new cases of AF-related stroke in the EU each year. Despite the known benefits of anticoagulation in AF and the availability of evidence-based guidelines, up to 40% of AF patients do not receive oral anticoagulant (OAC) therapy, she explained. Older patients, especially older women, are most likely to be under-treated.
There has been a widespread belief that aspirin provides an acceptable alternative to warfarin for patients who are unable to adhere to the complex dosage and monitoring requirements of warfarin. However, a 2007 meta-analysis of trials comparing aspirin with placebo for prevention of stroke in AF patients showed that it is ineffective. “I hope there is nobody in the audience who thinks that aspirin still has a role in preventing strokes in atrial fibrillation”, said Sotiris Antoniou (Consultant Cardiovascular Pharmacist, Barts Health NHS Trust, London). Warfarin treatment reduced stroke by 64% (compared with control or placebo). Moreover, when aspirin was directly compared with warfarin in elderly patients with AF (in the 2007 BAFTA study) warfarin was clearly superior and there was no difference in the risk of bleeding, showing conclusively that aspirin did not provide a safer way to prevent ischaemic strokes, he added.
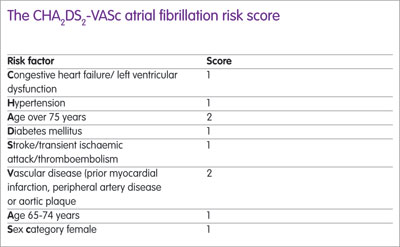
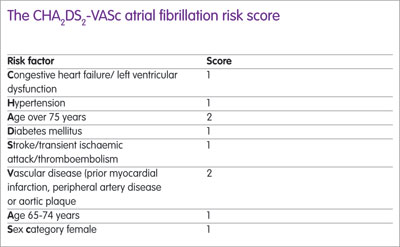
Four important trials have compared novel oral anticoagulants (NOACs) against vitamin K antagonists (VKAs) in patients with AF and a meta-analysis published in 2014 showed that NOAC treatment was significantly superior. The European Society of Cardiology recommends NOACs for anticoagulation (in preference to VKAs) for patients with a stroke risk (CHA2DS2-VASc score of 2 or more) (see Box) while the National Institute for Health and Care Excellence guidance recommends either a VKA or a NOAC, noted Mr Antoniou.
The uptake of NOACs across Europe has been variable, ranging from less than 5% of the total anticoagulant market in Hungary to more than 35% in Germany, Greece, Switzerland and Belgium, according to 2016 figures. When NOACs are prescribed, adherence has a critical impact on effectiveness; for every 10% decrease in adherence there is a 13% increase in the risk of mortality or stroke, explained Craig Coleman (Professor, University of Connecticut Evidence-based Practice Center, US). One large study involving more than 36,000 patients showed that about 30% were less than 80% adherent to NOAC treatment and in this group the risk of ischaemic stroke was 50% higher than in fully adherent patients.
A position statement has been drawn up by Ms Williams and Mr Antoniou describing how patients with AF should be managed. Service standards have been defined, for example, there should be opportunistic pulse checks of people over 65 years of age to identify undiagnosed AF, 80% of AF patients with high CHA2DS2-VASc scores should be anticoagulated, and patients who are poorly controlled on warfarin should be considered for NOAC treatment. “Pharmacists should be routinely checking patients’ pulses to identify people with AF”, said Ms Williams. They might also consider using a device that is attached to a smart phone and provides a single-lead ECG read-out. Such devices are inexpensive and are easy to use in day-to-day practice, she commented.
Ten years of biosimilars – what have we learned?
By February 2017, 31 biosimilar drugs had been approved in Europe and a further 14 were under review. The concept of a biosimilar drug that is highly similar but not identical to the originator product is well developed and the approval process is rigorous.
In practice, biosimilars have turned out to be remarkably safe drugs. There have been more than 400 million patient-days of exposure to biosimilars and there has been no evidence of clinical immunogenicity, according to Paul Cornes (Consultant Oncologist and member of the European Society for Oncology Task Force Advisory Group on Access to Innovative Treatment in Europe). He predicted a future scenario in which cancer treatment would be personalised, relying on identification and correction of deregulated (faulty) biochemical pathways using biological agents rather than chemotherapy. In order for this to be affordable, far greater use of biosimilars will be necessary, he argued. There is now growing acceptance of biosimilars amongst physicians and medical societies are beginning to reverse some of their previous “negative position statements”. For example, the British Society of Gastroenterology now recommends infliximab biosimilars as first-line anti-TNF-alpha treatment for active inflammatory bowel disease (IBD). Similarly, the European Crohn’s and Colitis Organisation now supports switching to biosimilars and, in 2017, EU regulators concluded that switching from the originator product to a biosimilar was safe.
Dr Cornes also noted that WHO has added filgrastim, trastuzumab and rituximab to the ‘essential drugs’ list and called for these drugs to be funded affordably. “We have gone from scepticism to confidence [with biosimilars]”, he concluded.
Questions of nomenclature and traceability of biosimilars remain important unresolved issues, according to Irene Krämer. For pharmacovigilance purposes it is essential to know exactly which product has been administered to a patient. Under the current system different biosimilar products have the same international non-proprietary name. The WHO has recognised that this is not satisfactory and has proposed the addition of a biological qualifier to distinguish between products, explained Professor Krämer. Clinicians are advised to document exactly which product is given to a patient and, when reporting suspected adverse reactions, both brand name and batch number are needed, she emphasised. Data from The Netherlands suggest that 76% of adverse reaction reports involving biologics include the brand name, but only 5% of reports include batch numbers. Barcoding of products would enable easy capture of this information in pharmacy-based aseptic preparation units, in wards and physicians’ offices. Where patients are self-administering these products, barcoded labels and a diary would be useful for accurate recording, she said.