Thrombotic thrombocytopenic purpura (TTP) is an acute, rare, thrombotic microangiopathy, which is diagnosed by the presence of thrombocytopenia and non-immune haemolysis, associated with anaemia, raised reticulocyte count and lactate dehydrogenase levels, and often an increased unconjugated bilirubin.
It has specific blood film changes, specifically, the presence of fragmented red blood cells and polychromasia, signifying the shearing of red blood cells over microthrombi in states of high shear and the early release of new red blood cells from the bone marrow.1
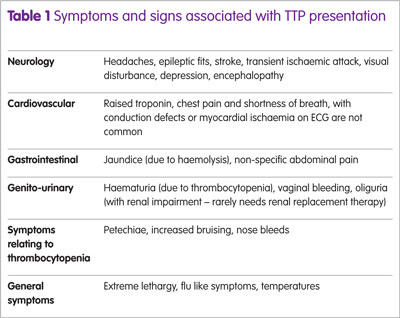
TTP is a very rare condition resulting from a severe deficiency of a metalloproteinase enzyme, ADAMTS 13, which is required for the cleavage of von Willebrand factor. In acquired, immune-mediated TTP, antibodies, primarily IgG, to ADAMTS 13 are detected. Failure of VWF cleavage results in accumulation of platelets on large and ultra-large VWF fragments. These detach, forming microvascular thrombi in multiple organs. At a critical level, symptoms may be evident, primarily neurological, renal impairment, abdominal pain and cardiac involvement (Table 1).
There are many conditions that may present with a microangiopathic haemolytic anaemia and thrombocytopenia, the most common being disseminated intravascular coagulation (DIC), and also, complement-mediated haemolytic uraemic syndrome (CM HUS), which more likely presents with an acute kidney injury requiring renal replacement.
Treatment for TTP requires prompt initiation once the diagnosis is considered, because without it, mortality is 90%. The replacement of the missing enzyme, through plasma exchange (PEX) using plasma containing ADAMTS 13 is required as is removal of antibodies to ADAMTS 13, aided by PEX, and also pharmacological immunosuppressive/immunomodulatory therapy.
PEX
One of the few clinical trials performed in the early 1990s demonstrated the improved survival of 80% in patients with TTP, if given PEX rather than plasma infusion (PI).2 The type of plasma used varies between countries and institutions. In the main, standard FFP is used. In the UK, non-UK sourced solvent detergent plasma with an addition prion-reducing step is used (Octaplas LG) on the basis of preventing prion-mediated disease.3 All plasma-containing products have an equivalent quantity of ADAMTS 13 measured in vitro.4 However, the risk of reactions is considerably reduced with S/D plasma (Octaplas) compared with standard FFP,5 which is important at reducing morbidity but also has potential in preventing an exacerbation of disease.
Acute steroid use
There are limited data on the use of steroids in TTP, but this practice has been empirical and justified, since the identification of antibodies to ADAMTS 13. One of the largest historic series of 108 patients, using high dose prednisolone and PEX, demonstrated the first convincing evidence of the benefit of steroids, even before the identification of a missing enzyme required to cleave VWF.6 Subsequent experience has confirmed the initial excellent response rates with steroids, but the risk of exacerbation at 30 days is 60%.7 Only one study has compared high- versus standard-dose methylprednisolone. There was a significant difference in failure to achieve complete remission (CR) by day 23 and a non-significant difference regarding failure to achieve a good response by day 9 and death against standard dose methylprednisolone.8
Rituximab therapy
The greatest advance has been the use of rituximab or anti-CD20 therapy, initially in acute refractory/relapsing TTP9-11 and subsequently in the acute phase, that is, within three days of presentation. Earlier use of rituximab is associated with reduced PEX to CR, reduced in-patient stay and reduced time to CR from admission. Furthermore, a surprising finding from the early use of rituximab was a reduced relapse rate, previously recorded as 30–50% of all immune TTP cases.12
Rituximab used during PEX will be removed during the apheresis procedure. Indeed, 65% of the monoclonal antibody is removed per procedure and, normally given weekly in other disease protocols such as lymphoma, it was identified that the trough levels at 7 days are almost undetected. For this reason, while patients are undergoing PEX, they receive rituximab every 3 to 4 days.13 Furthermore, once rituximab is give, at least four hours is required for distribution, but preferably, depending on the patients clinical condition, 24 hours is left between further PEX.
Other immune suppressive therapy
A number of other treatments have been suggested and used over the years, including vincristine, which is thought to prevent platelet binding to endothelia,14 and ciclosporin15 and cyclophosphamide.16 The role of splenectomy in TTP, or indeed in other autoimmune thrombocytopenic conditions, remains controversial. There is a potential benefit of splenectomy, which is generally used in severe refractory patients not responding to above documented therapies.17 More recently, there are case reports/small series of the use of bortezimib in acute TTP.18 Its action is thought to be inhibition of plasma cells and possibly antigen-presenting cells. This is relevant as no other therapies currently target plasma cells, including rituximab. While the proteasome inhibitor bortezimib has shown to be beneficial in a number of severe and refractory cases, it should be used with caution. Proteasome inhibitors, have a cardiac signal. This is relevant regarding early use in TTP, because 50% of patients have raised troponin levels associated with microvascular myocardial damage.
Adjunct treatments
NAC (N acetyl cysteine) has been used for many years in non-haematological conditions, most notably, paracetamol overdose. However, NAC in vitro has been shown to inhibit platelet binding to ultra-large VWF multimers, so reducing platelet adhesion.19 However, despite this, there are limited case reports,20 but it does appear to provide an organ protective effect, while PEX and immunosuppressives act to reduce ADAMTS 13 antibodies.
The benefit of rituximab in achieving remission and preventing relapse of TTP has been discussed. However, there is a median period of ten days before it achieves CR.21 Furthermore, mortality and disease-related morbidity of TTP, particularly in the acute period, is significant. Caplacizumab is a new therapeutic, belonging to a new class of drugs. The nanobody, derived from the Camelid family (llamas etc), is a small single arm heavy chain molecule, and it specifically binds to the A1 region of VWF and prevents platelet binding.
Therefore, it prevents development of further microthrombi, and release of platelets is associated with a faster increase to normalisation of the laboratory measured platelet count. This has been shown in the Phase II TITAN trial.22 The immediate use of caplacizumab with confirmation of a diagnosis of TTP would hope to support prevention of organ damage, reduce acute mortality and bridge the period required for immunotherapy to reduce ADAMTS 13 IgG antibodies and result in normalisation of ADAMTS 13 activity. If these end points are achieved, they will be presented with the results of the Phase III trial in acute, immune-mediated TTP: namely, HERCULES.
Future developments
PEX remains a critical component of therapy in acute TTP, but is not without its risks, including central venous catheter insertion. The use of plasma therapy, regardless of safety profile, still has a risk of allergic reactions or anaphylaxis and risks of unknown pathogen transmission. Furthermore, there are components other than ADAMTS 13 present in plasma, unnecessary for disease remission. Recombinant ADAMTS 13, a lyophilised pure enzyme replacement, has been trialled in congenital TTP and further Phase II and III trials are expected in congenital and immune-mediated disease. Confirmation of safety and efficacy will transform treatment pathways for acute TTP patients.
Conclusions
In conclusion, we are entering an era of bespoke treatment and simplification of patient pathways, aiming to improve mortality and reduce the morbidity associated with this life-threatening disorder. Recombinant ADAMTS 13 enzyme replacement, use of nanobodies to protect and bridge while monoclonal antibody therapy reduces anti-ADAMTS 13 antibodies and restore normal enzyme activity levels are some of the therapeutic options.
Key points
- Thrombotic thrombocytopenic purpura is an acute life-threatening condition, requiring prompt diagnosis and treatment.
- Plasma exchange remains the mainstay of therapy to replenish the missing enzyme, ADAMTS.13
- Immunospuppression to reduce ADAMTS 13 IgG autoantibodies is required.
- Rituximab is associated with disease remission and clearance of antibodies to ADAMTS.13
- Newer therapies are undergoing clinical trials, including the nanobody, caplacizumab, and recombinant ADAMTS.13
References
1 Scully M et al. Guidelines on the diagnosis and management of thrombotic thrombocytopenic purpura and other thrombotic microangiopathies. Br J Haematol 2012;158:323–35.
2 Rock GA et al. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. Canadian Apheresis Study Group. N Engl J Med 1991;325:393–7.
3 Vendramin C et al. A single-center prospective study on the safety of plasma exchange procedures using a double-viral-inactivated and prion-reduced solvent/detergent fresh-frozen plasma as the replacement fluid in the treatment of thrombotic microangiopathy. Transfusion (Paris) 2017;57:131–6.
4 Yarranton H et al. Comparison of von Willebrand factor antigen, von Willebrand factor-cleaving protease and protein S in blood components used for treatment of thrombotic thrombocytopenic purpura. Transfus Med 2004;14:39–44.

5 Scully M et al. Cryosupernatant and solvent detergent fresh-frozen plasma (Octaplas) usage at a single centre in acute thrombotic thrombocytopenic purpura. Vox Sang 2007;93:154–8.
6 Bell WR et al. Improved survival in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Clinical experience in 108 patients. N Engl J Med 1991;325:398–403.
7 Cataland SR et al. Cyclosporin and plasma exchange in thrombotic thrombocytopenic purpura: long-term follow-up with serial analysis of ADAMTS13 activity. Br J Haematol 2007;139:486–93.
8 Balduini CL et al. High versus standard dose methylprednisolone in the acute phase of idiopathic thrombotic thrombocytopenic purpura: a randomized study. Ann Hematol 2010;89:591–6.
9 Scully M et al. Remission in acute refractory and relapsing thrombotic thrombocytopenic purpura following rituximab is associated with a reduction in IgG antibodies to ADAMTS-13. Br J Haematol 2007;136:451–61.
10 Fakhouri F et al. Efficiency of curative and prophylactic treatment with rituximab in ADAMTS13-deficient thrombotic thrombocytopenic purpura: a study of 11 cases. Blood 2005;106:1932–7.
11 Froissart A et al. Efficacy and safety of first-line rituximab in severe, acquired thrombotic thrombocytopenic purpura with a suboptimal response to plasma exchange. Experience of the French Thrombotic Microangiopathies Reference Center. Crit Care Med 2012;40:104–11.
12 Scully M et al. A phase 2 study of the safety and efficacy of rituximab with plasma exchange in acute acquired thrombotic thrombocytopenic purpura. Blood 2011;118:1746–53.
13 McDonald V et al. Rituximab pharmacokinetics during the management of acute idiopathic thrombotic thrombocytopenic purpura. J Thromb Haemost 2010;8:1201–8.
14 Bohm M et al. The course of ADAMTS-13 activity and inhibitor titre in the treatment of thrombotic thrombocytopenic purpura with plasma exchange and vincristine. Br J Haematol 2005;129:644–52.
15 Cataland SR et al. An evaluation of cyclosporin and corticosteroids individually as adjuncts to plasma exchange in the treatment of thrombotic thrombo-cytopenic purpura. Br J Haematol 2007;136:146–9.
16 Beloncle F et al. Splenectomy and/or cyclophosphamide as salvage therapies in thrombotic thrombocytopenic purpura: the French TMA Reference Center experience. Transfusion (Paris) 2012;52:2436–44.
17 Schaller M et al. The splenic autoimmune response to ADAMTS13 in thrombotic thrombocytopenic purpura contains recurrent antigen-binding CDR3 motifs. Blood 2014;124:3469–79.
18 Patriquin CJ et al. Bortezomib in the treatment of refractory thrombotic thrombocytopenic purpura.
Br J Haematol 2016;173(5):779–85.
19 Li GW et al. Treatment of refractory thrombotic thrombocytopenic purpura with N-acetylcysteine: a case report. Transfusion (Paris) 2014;54:1221–4.
20 Rottenstreich A et al. The role of N-acetylcysteine in the treatment of thrombotic thrombocytopenic purpura. J Thromb Thrombolysis 2016;41:678–83.
21 Westwood JP et al. Rituximab for thrombotic thrombocytopenic purpura: benefit of early administration during acute episodes and use of prophylaxis to prevent relapse. J Thromb Haemost 2013;11:481–90.
22 Peyvandi F et al. Caplacizumab for acquired thrombotic thrombocytopenic purpura. N Engl J Med 2016;374:511–22.
