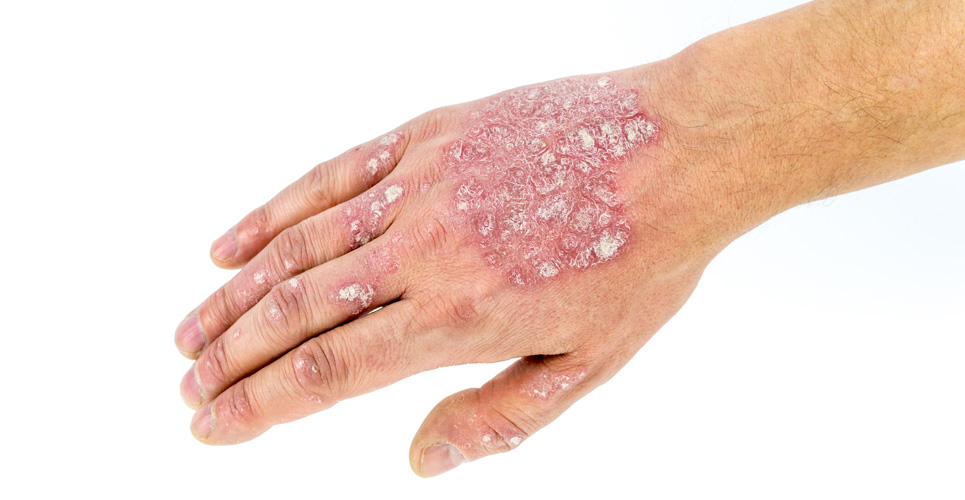
Novartis announced new late-breaking data from the head-to-head CLEAR study, demonstrating that Cosentyx (secukinumab) remains superior to Stelara (ustekinumab) in achieving sustained skin clearance (PASI 90 response) at 52 weeks for adults living with moderate-to-severe psoriasis. These findings were presented for the first time at the American Academy of Dermatology (AAD) Annual Meeting in Washington, DC.1
Novartis announced new late-breaking data from the head-to-head CLEAR study, demonstrating that Cosentyx (secukinumab) remains superior to Stelara (ustekinumab) in achieving sustained skin clearance (PASI 90 response) at 52 weeks for adults living with moderate-to-severe psoriasis. These findings were presented for the first time at the American Academy of Dermatology (AAD) Annual Meeting in Washington, DC.1
Cosentyx is the first fully human interleukin-17A inhibitor approved for adults to treat moderate-to-severe plaque psoriasis, and was also recently approved for the treatment of psoriatic arthritis and ankylosing spondylitis in Europe.
“Cosentyx continues to demonstrate superior and sustainable efficacy against currently available biologics and is a proven first-line treatment option for adult patients with moderate-to-severe psoriasis,” said Vasant Narasimhan, Global Head, Drug Development and Chief Medical Officer, Novartis. “Cosentyx has the potential to give more people with psoriasis than ever before the benefit of long-lasting skin clearance.”
The ultimate aim of psoriasis treatment is clear skin, and the Psoriasis Area Severity Index (PASI) 90 response is considered an important measure of treatment success.2,3 Meeting all primary and secondary endpoints at Weeks 4, 16 and 52, Cosentyx demonstrated it remains consistently superior to Stelara in achieving and sustaining PASI 90 response (76.2% versus 60.6%; P<0.0001), and significantly better in achieving PASI 100 (clear skin) response (45.9% versus 35.8%; P=0.0103) at 52 weeks.1,4 Cosentyx also showed significantly greater and sustained Dermatology Life Quality Index (DLQI) 0/1 responses versus Stelara (71.6% versus 59.2%; P=0.0008) at 52 weeks.1
The study also demonstrated Cosentyx had a superior rapid onset of action compared to Stelara, with half of Cosentyx patients achieving PASI 75 as early as Week 4 (50.0% versus 20.6%, P<0.0001).4 Cosentyx had a similar safety profile to that of Stelara in the study, which was consistent with that reported in the pivotal Cosentyx Phase III studies.1
Psoriasis is a chronic inflammatory condition, which is estimated to affect between 2% and 3% of the UK population – up to 1.8 million people.5 Psoriasis causes itching, scaling and pain, and can have a significant impact on physical and psychological wellbeing.6 Despite this, up to half of patients receive no treatment, and of those who do, many (52%) remain dissatisfied with their disease management.8
References
- Blauvelt A et al. Secukinumab demonstrates superior sustained efficacy vs. ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: 52-week results from the CLEAR study. 74th Annual Meeting of the American Academy of Dermatology, 4–8 Mar 2016, Washington DC (oral presentation by D Thaçi on 5 Mar 2016).
- Guideline on clinical investigation of medicinal products indicated for the treatment of psoriasis 2004. European Medicines Agency website. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003329.pdf. Last accessed March 2016.
- Ryan C et al. Research gaps in psoriasis: opportunities for future studies. J Am Acad Dermatol 2014;70:146–67.
- Thaçi D et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol 2015 [E-pub ahead of print].
- Psoriasis Association. About Psoriasis. https://www.psoriasis- association.org.uk/pages/view/about-psoriasis. Last accessed March 2016.
- International Federation of Psoriasis Associations (IFPA) World Psoriasis Day website. “About Psoriasis.” Available at: http://www.worldpsoriasisday.com/web/page.aspx?refid=114. Last accessed March 2016.
- Armstrong AW et al. Under treatment, treatment trends, and treatment dissatisfaction among patients with psoriasis and psoriatic arthritis in the United States: findings from the National Psoriasis Foundation surveys, 2003-2011. JAMA Dermatol 2013;149(10):1180–5.
- Mease PJ, Armstrong AW. Managing patients with psoriatic disease: the diagnosis and pharmacologic treatment of psoriatic arthritis in patients with psoriasis. Drugs 2014;74:423–41.