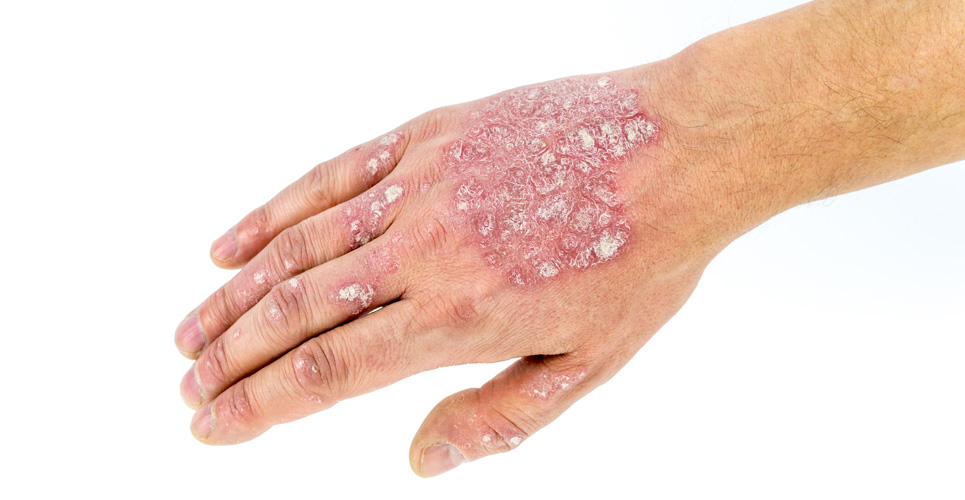
Novartis has announced that new Phase III results from its expanding specialty dermatology portfolio will be presented at the 22nd Congress of the European Association of Dermatology and Venereology (EADV), taking place in Istanbul, Turkey, from 2-6 October 2013.
Pivotal data from Phase III studies of secukinumab (AIN457) in moderate-to-severe plaque psoriasis and omalizumab (Xolair®) in chronic spontaneous urticaria (CSU) are among the landmark Novartis results being presented for the first time at the congress.
“The new Phase III results for secukinumab and omalizumab being presented at EADV show our potential to transform patient care in psoriasis and CSU, with the aim of helping patients to achieve clear skin,” said David Epstein, Head of the Pharmaceuticals Division of Novartis Pharma AG. “Specialty dermatology is an emerging area of importance for Novartis, and we are on track to deliver an innovative approach to targeted therapies for people suffering from severe skin diseases in need of new treatment options.”
Specifically, results from the pivotal head-to-head Phase III FIXTURE study showing the superiority of the IL-17A inhibitor secukinumab to Enbrel®* (etanercept), part of a group of medications known as anti-TNF inhibitors, in moderate-to-severe plaque psoriasis will be presented for the first time at EADV. Data from three additional Phase III studies from the robust, global secukinumab clinical trial program – the largest undertaken in moderate-to-severe plaque psoriasis to date – will also be revealed at the congress.
In addition, data from ASTERIA I, the final of three pivotal registration studies for omalizumab in CSU, will be presented for the first time at EADV. Positive and consistent results from ASTERIA II and GLACIAL, the two other Phase III studies for omalizumab in CSU, were presented at major medical meetings earlier this year. Omalizumab is currently not approved for the treatment of CSU.
Secukinumab is the first therapy selectively targeting IL-17A to present Phase III results in moderate-to-severe plaque psoriasis. IL-17A is a central cytokine (messenger protein) involved in the development of psoriasis, and is found in high concentration in skin affected by the disease.[1,2] Research shows that IL-17A plays a key role in driving the body’s autoimmune response in disorders such as moderate-to-severe plaque psoriasis and is a preferred target for investigational therapies.[3-7] There is a clear unmet need for new psoriasis therapies that act faster and longer to relieve the debilitating symptoms of the disease.[8-12]
CSU, also known as chronic idiopathic urticaria (CIU) in the US, is a severe and distressing skin condition characterised by red, swollen, itchy and sometimes painful hives or wheals on the skin[13,14] that spontaneously present and re-occur for more than six weeks.[15] At any given time the prevalence of CSU is 0.5% to 1% worldwide.[16] There is also a significant unmet need in CSU, as more than 50% of patients do not respond to approved doses of antihistamines, the basis of current symptomatic therapy.[17]
References
- Johansen C, Usher PA, Kjellerup RB, et al. Characterization of the interleukin-17 isoforms and receptors in lesional psoriatic skin Brit J Dermatol. 2009; 160:319-24.
- Arican O, Aral M, Sasmaz S, et al. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005; Oct 24;2005(5):273-9.
- Gaffen SL. Structure and signaling in the IL-17 receptor family. Nat Rev Immunol. 2009; 9(8):556-67.
- Ivanov S, Linden A. Interleukin-17 as a drug target in human disease. Trends Pharmacol Sci. 2009; 30(2):95-103.
- Kopf M, Bachmann MF, Marsland BJ. Averting inflammation by targeting the cytokine environment. Nat Rev Drug Discov. 2010; 9(9):703-718.
- Onishi RM, Gaffen SL. Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology. 2010; 129(3):311-321.
- Krueger J, Fretzin S, Suárez-Fariñas M, et al. IL-17A is essential for cell activation and inflammatory gene circuits in subjects with psoriasis. J Allergy Clin Immunol. 2012; 130(1):145-154.
- Krueger JG, Koo J, Lebwohl M, et al. The impact of psoriasis on quality of life: Results for a 1998 National Psoriasis Foundation patient membership survey. Arch Derm. 2001; 137:280-284.
- Mason AR, Mason J, Cork M et al. Topical treatments for chronic plaque psoriasis. Cochrane Database Syst Rev. 2009;15;(2):CD005028.
- Langley R G B, Krueger G G, Griffiths C E M. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005; 64(Suppl II):ii18-ii23.
- Ljosaa TM, Mork C, Stubhaug A, et al. Skin pain and skin discomfort is associated with quality of life in patients with psoriasis. J Eur Acad Dermatol Venereol. 2012;26:29-35.
- Sterry W, Barker J, Boehncke WH, Bos JD, Chimenti S, Christophers E, De La Brassinne M, Ferrandiz C, Griffiths C, Katsambas A, Kragballe K, Lynde C, Menter A, Ortonne JP, Papp K, Prinz J, Rzany B, Ronnevig J, Saurat JH, Stahle M, Stengel FM, Van De Kerkhof P, Voorhees J. Biological therapies in the systemic management of psoriasis: International Consensus Conference. Br J Dermatol. 2004 Aug;151 Suppl 69:3-17.
- Asthma and Allergy Foundation of America (AAFA) website. “Chronic Urticaria (Hives).” http://www.aafa.org/display.cfm?id=9&sub=23&cont=328. Accessed November 14, 2012.
- American Academy of Allergy Asthma & Immunology (AAAAI) website. “Skin Allergy Overview.” http://www.aaaai.org/conditions-and-treatments/allergies/skin-allergy.aspx. Accessed November 14, 2012.
- Maurer M, Rosén K, Hsieh HJ, et al. Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. NEJM 2013; DOI: 10.1056/NEJMoa1215372.
- Maurer M, Weller K, Bindslev-Jensen C, et al. Unmet clinical needs in chronic spontaneous urticaria. A GA2LEN task force report. Allergy 2011; 66: 317-330.
- Kaplan A, Ledford D, Ashby M, et al. Omalizumab in patients with symptomatic chronic idiopathic/spontaneous urticaria despite standard combination therapy. J Allergy Clin Immunol. 2013 Jul;132(1):101-9. doi: 10.1016/j.jaci.2013.05.013.