The National Institute for Health Care and Excellence (NICE) has issued a Final Appraisal Determination (FAD) not recommending OTEZLA® (apremilast), a novel oral treatment for adults with moderate to severe plaque psoriasis, for use within NHS England and Wales. (1)
The National Institute for Health Care and Excellence (NICE) has issued a Final Appraisal Determination (FAD) not recommending OTEZLA® (apremilast), a novel oral treatment for adults with moderate to severe plaque psoriasis, for use within NHS England and Wales. (1)
The FAD recognises OTEZLA as an innovative step forward in psoriasis treatment and concluded that it offers clinical benefits to patients. Despite this, the incremental cost-effectiveness ratios (ICERs) for OTEZLA in moderate to severe psoriasis were not within the range considered to be a cost-effective use of NHS resources. NICE considered the most plausible ICER available for the OTEZLA sequence (in which OTEZLA was positioned before biological therapies) was about £30,300 per quality-adjusted life years (QALY) gained. (1)
Professor Mark Goodfield, Consultant Dermatologist at Leeds Teaching Hospitals NHS Trust, added: “NICE’s decision to not recommend apremilast is very disappointing for patients and their doctors. Having participated in the apremilast clinical trial programme, I have seen first-hand the difference this oral treatment can make, particularly in hard to treat areas such as nails and scalp, and for problem symptoms such as itch. Apremilast has the potential to fill an important gap in the treatment pathway. This negative decision denies people in England and Wales a treatment option available elsewhere in the world.”
Unlike current systemic treatments available for psoriasis, OTEZLA does not require regular laboratory monitoring, (2,3) which may help reduce the burden of hospital visits for patients. OTEZLA works by reducing the activity of an enzyme called phosphodiesterase 4 (PDE4). PDE4 has been implicated in the process of inflammation. By reducing the activity of this enzyme, OTEZLA can help to modulate the inflammatory process associated with psoriasis. (4,5)

In clinical trials, treatment with OTEZLA for psoriasis showed a reduction in psoriatic skin plaques and other signs and symptoms of the disease including itch, skin pain and discomfort. (2,6) OTEZLA also showed improvements in nail and scalp psoriasis. (2,6,7) and the quality of life in patients with psoriasis. (2) Most adverse reactions were considered to be mild or moderate in severity. Gastrointestinal (GI) symptoms including nausea and diarrhoea were the most commonly reported adverse reactions in the Phase III clinical studies. (2)
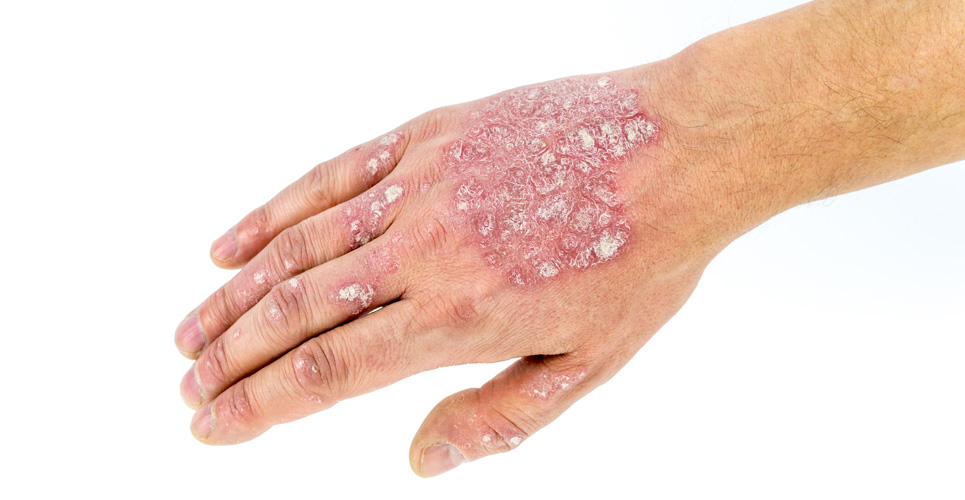
Psoriasis, an inflammatory disease of the skin, (8) is estimated to affect at least 830,000 adults the in the UK. (9,10) Despite there being a number of effective treatment options available for psoriasis, evidence suggests that a substantial proportion of people with psoriasis recognise the need for new treatment. (11)
Celgene’s Medical Director for Inflammation and Immunology, Dr Dani Thomas, added: “Celgene is disappointed at the Final Appraisal Determination outcome but we are committed to working through the current issues with NICE and will continue to explore potential options to facilitate access to OTEZLA for people with psoriasis and psoriatic arthritis in England, Wales and Northern Ireland.”
The Scottish Medicines Consortium (SMC) issued positive advice for OTEZLA for both chronic plaque psoriasis and active psoriatic arthritis in June 2015. (12,13) In August, NICE issued a negative final recommendation for OTEZLA for the treatment of active psoriatic arthritis. (14)
“This decision is a stark reminder of the post code lottery that exists within the UK,” added Dr Dani Thomas. “People living with psoriasis and psoriatic arthritis in Scotland will be able to benefit from this treatment, yet over the border in England and Wales, patients have been denied access to OTEZLA for both these conditions.”
Please click here for the Summary of Product Characteristics.
References:
- Final Appraisal Determination – apremilast for moderate to severe chronic plaque psoriasis. NICE. 2015.
- OTEZLA® Summary of Product Characteristics. Current version available online at www.medicines.org.uk.
- Reich K et al. Long-term Safety and Tolerability of Apremilast, an Oral Phosphodiesterase 4 Inhibitor, in Patients With Moderate to Severe Psoriasis: Results From a Phase III, Randomized, Controlled Trial (ESTEEM 1). P8296. Presented at the 72nd Annual Meeting of the American Academy of Dermatology 2014; March 21-25;Denver, CO, USA.
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol 2012;83:1583–90.
- Schafer PH et al. Apremilast, a cAMP phosphodiesterase-4 inhibitor, demonstrates anti-inflammatory activity in vitro and in a model of psoriasis. Br J Pharmacol 2010;159:842–55.
- Yosipovitch G et al. Effects of apremilast on pruritus in patients with moderate to severe plaque psoriasis: Results from the ESTEEM 1 and 2 trials. Abstract P1682. Presented at the 23rd Congress of the European Academy of Dermatology and Venereology 2014; October 8–12; Amsterdam, the Netherlands.
- Crowley J et al. Apremilast in Moderate to Severe Plaque Psoriasis: 32-Week Results in Patients With Nail, Scalp, and Palmoplantar Involvement (ESTEEM 2). Abstract P1687. Presented at the 23rd Congress of the European Academy of Dermatology and Venereology 2014; October 8-12; Amsterdam, the Netherlands.
- Racz E et al. GATA3 expression is decreased in psoriasis and during epidermal regeneration; induction by narrow-band UVB and IL-4. PLoS One. 2011;6:e19806.
- Parisi R et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol 2013;133(2):377–85 .
- Office for National Statistics. Annual Mid-year Population Estimates, 2013. Available from: http://www.ons.gov.uk/ons/dcp171778_367167.pdf. Last accessed May 2015.
- Lebwohl MG et al. Patient perspectives in the management of psoriasis: Results from the population-based multinational assessment of psoriasis and psoriatic arthritis survey. J Am Acad Dermatol 2014; 70(5): 871–81.
- Psoriasis DAD Document. Available from: https://www.scottishmedicines.org.uk/files/advice/apremilast__Otezla__plaque_psoriasis_FINAL_May_2015_REVISED_010615_for_website.pdf. Last accessed Aug 2015.
- PsA DAD Document. Available from: https://www.scottishmedicines.org.uk/SMC_Advice/Advice/1053_15_apremilast_Otezla_psoriatic_arthritis/apremilast_Otezla_psoriatic_arthritis. Last accessed August 2015.
- Psoriatic arthritis FAD Document. Available from: http://www.nice.org.uk/guidance/gid-tag468/resources/psoriatic-arthritis-active-apremilast-post-dmards-id682-final-appraisal-determination-document2. Last accessed September 2015.