teaser
US regulators have approved the use of Amgen’s osteoporosis drug Prolia in post-menopausal women.
The Food and Drug Administration (FDA) took the decision on the basis of a study, which showed the medication significantly reduced the incidence of vertebral, non-vertebral, and hip fractures in the target group.
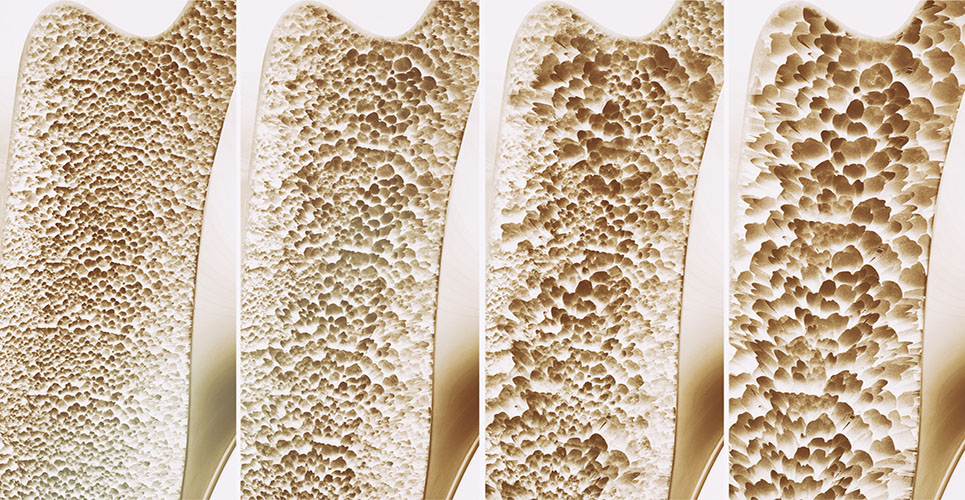
Prolia, also known as denosumab, strengthens bone mass and density by inhibiting proteins that work to activate destructive cells.
The treatment is given by injection once every six months, and thus may prove appealing to patients who are unable to tolerate regular drug doses.
The news follows an identical decision from the European Medicines Agency last week.
Roger Perlmutter, Amgen’s research chief, said: “Denosumab is the most potent agent ever introduced into clinical practice that blocks bone degradation.”
The National Institute of Arthritis and Musculoskeletal and Skin Diseases estimates that one out of every two women over the age of 50 will break a bone in their lifetime because of osteoporosis.
Copyright Press Association 2010
Amgen