Stelara has been approved for use in line with anti-tumour necrosis factor (TNF)-alpha therapies for the treatment of psoriatic arthritis in Europe.
This means that physicians and patients now have an additional treatment choice with a different mode of action to help them manage psoriatic arthritis.
Stelara has been approved for use in line with anti-tumour necrosis factor (TNF)-alpha therapies for the treatment of psoriatic arthritis in Europe.
This means that physicians and patients now have an additional treatment choice with a different mode of action to help them manage psoriatic arthritis.
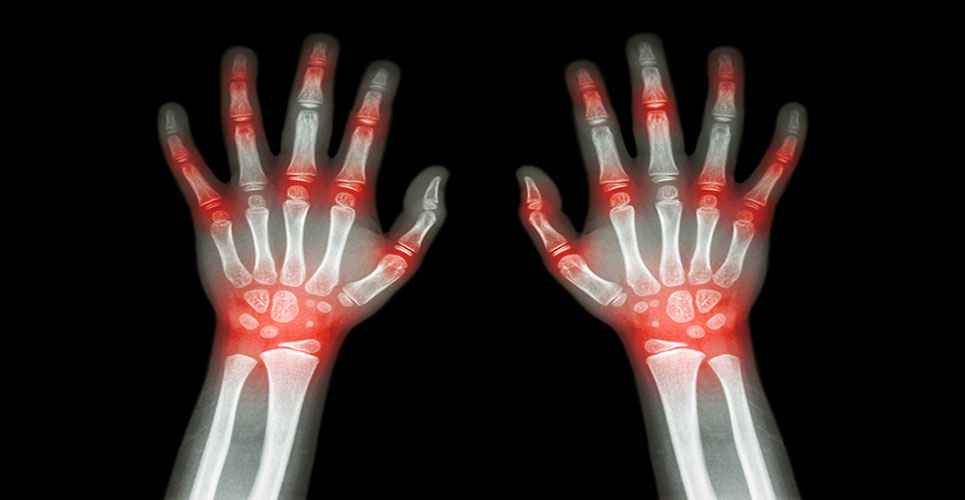
The label enhancement, supporting the effect of Stelara in reducing the rate of progression of structural damage, represents an important milestone for people living with psoriatic arthritis. Approximately 14 million people in Europe are affected by psoriasis. Over time, up to 30% of patients suffering from psoriasis will develop psoriatic arthritis.
This label enhancement follows the publication of data from two pivotal phase III studies, PSUMMIT I and II. The data supported the effect of Stelara in reducing the rate of progression of structural damage in the treatment of active psoriatic arthritis and demonstrated significant and sustained improvements in reducing the signs and symptoms of psoriatic arthritis, physical function and health related quality of life over 2 years.
Stelara now has data to support its efficacy over a broad range of clinical features of psoriatic arthritis and the Summary of Product Characteristics (SmPC) has been updated to reflect this.
New psoriatic arthritis data from the PSUMMIT I and II studies is due to be presented at the forthcoming European League Against Rheumatism (EULAR) 2014 congress in Paris (11–14 June).