David Upton PhD MRPharmS
David Upton PhD MRPharmS
Medication Safety Pharmacist and Clinical Director,
Pharmacy & Medicines Management,
Sheffield Children’s NHS Foundation Trust
Sheffield, UK
Email: [email protected]
Since the introduction of dose error reduction systems (DERS) to the European market in 2002, anecdotal feedback from adopters of the technology has indicated that one of the main hurdles to overcome when implementing ‘smart pumps’ is the development and agreement of standardised drug concentrations and infusion alert levels for each drug in each clinical profile area. It is clear that clinical differences make it extremely difficult to utilise a ‘one fit for all’ drug library, even across discreet, yet similar, clinical areas such as critical care or neonatal units. This article explores some of the barriers to implementing such a system in the UK and recommends a number of drug templates to expedite DERS implementation. It should be noted that this problem is not unique to the UK and in reality poses an international challenge.
Background
Medication safety and management, especially when administering injectable medicines, is a well-documented problem throughout all international healthcare systems.(1–6) These reports and studies range from the landmark publication ‘To Err is Human’ from the US Institute of Medicine, to an Australian publication, over 12 years later, that highlights many of the issues around IV administration previously mentioned in the US report. From this it could be concluded that, although there has been much learning internationally around medication management and safety, similar errors continue to occur
In a survey of medication errors,(7) a number of factors contributing to medication adverse drug events included multiple formulations of the same drug leading to confusion, confusion caused by packaging and labelling, dosing errors (for example, misplaced decimal points/zeros, calculation errors), drug administration errors (for example, omission, wrong route, given too rapidly) and lack of knowledge or competence (for example, about drugs, formulations and procedures).
Since the turn of the millennium a number of solutions across the range of healthcare settings have been introduced to address factors contributing specifically to injectable medication error. Solutions include standardisation of equipment,(8) the use of ‘smart pumps’ with DERS,(9) recommendations for injectable medicines(10) and the promotion of drug concentration standardisation.(11) Each of these solutions attempts to address specific issues associated with injectable drug delivery. This also illustrates how complex the problem of medication risk management can be and that the answer does not lie in just one solution, multiple practices and standards being required.(12)
While many of the above solutions have a unique focus, there is also a significant amount of overlap between them. The key focus of this article is to consider the synergies between the two significant solutions: drug concentration standardisation and the use of DERS via ‘smart pumps’.
DERS with ‘smart pumps’
The DERS provides a final ‘test of reasonableness’ that can prevent an incorrect dose from being administered to the patient accidentally.(13) The system can support clinical area profiles that enable pumps to be configured differently for specific patient groups in terms of the individual drug used, the dosing units used, the maximum patient weight permissible, the maximum volume to be infused and the maximum rate of the infusion.(9)
The DERS system is a software program that sits inside the infusion device and recognises when a deviation from agreed pre-set limits has been attempted. Each infusion device contains a library of standardised drug concentrations and each drug concentration can have individual pre-determined limits programmed. Limits are established in order to prevent under- or overdosing of medications and can be set as ‘hard’, in which case they cannot be overridden, or ‘soft’, where the limit alert can be overridden. The user is then alerted via the infusion device alarm alert when a limit is reached.
A review of DERS(14) suggested that: ‘the impact (of introducing a DERS) could result in changes to drug preparation and distribution practices. Drug preparation, for example, could become more centralised in pharmacy departments, with practical and cost advantages. Alternatively, remote drug preparation may not always be ideal because of delays in the availability of medicines, which may result in adverse outcomes for patients’.
Many DERS systems record these events in their memory, which can then be downloaded, analysed and reported to assist users in learning from infusion practice problems.
Because many more potentially high-risk drugs are given in critical care areas than in other areas, and because most drugs are given via IV infusion, medication error rates tend to be higher in ICU.(15) For these reasons, it is common practice for the introduction of DERS into healthcare institutions to begin with critical care areas.
Managing the introduction of DERS with ‘smart pumps’
There are a number of important steps that must be considered to ensure that DERS works effectively. It is vital that key stakeholders be involved in the development and implementation process.(16) The group should comprise clinical leads (medical and nursing), pharmacists, medical equipment manager, risk manager, training leads and the infusion device provider (supplier) team. One of the first, and most significant, steps towards medication error reduction is the dialogue and team review, which is the starting point for the drug library development.
The stakeholder team should review drug protocols and establish standardised drug libraries. This critical process can often uncover significant discrepancies in drug usage and administration but is often one of the main stumbling blocks when trying to agree on standard concentrations.
Promoting standardised concentrations
The need to standardise drug concentrations as a safety measure is recognised internationally and well documented. In the UK there are a number of initiatives that promote standard concentrations. The UK Injectable Medicines Guide(17) provides a range of information for the use of injectable medicines and standard concentrations, and is accessed by the majority of UK National Health Service (NHS) hospitals. Contained in this website is guidance for the use of injectable medicines for paediatrics as well as adults, and the website centralises work from specific organisations, for example, the Intensive Care Society (ICS). The ICS found that there was wide variation in infusion practice in UK critical care units and, as a consequence, now supports and promotes the adoption of standard infusion concentrations through a list of concentrations endorsed by the ICS Standards Committee.(18)
Standardising infusion concentrations may lead to safety and efficiency gains through reduced training burdens, common nomenclature, reductions in preparation and calculation error rates and facilitation of mass production of ready-to-use products by the pharmaceutical industry.(11,19)
In the USA, the Institute for Safe Medication Practice (ISMP) and Vermont Oxford Network collaborated to identify and promote the standardisation of concentrations of typical neonatal drug infusions.(20) The safety benefits associated with the use of standard concentrations were cited as:
- A reduction of medication error when critically ill neonates are transferred from one facility to another
- Stimulates the development of standardised infusion device drug libraries
- Provides the demand necessary for manufacturers to offer commercially prepared standard solutions (if not already available), thereby reducing the risk of extemporaneous compounding errors within hospitals.
Barriers to implementation
The introduction of new technologies can present a challenge to existing clinical practices, including potential workflow disruption,(21) but they may also save time and free staff to do other tasks, which could be expected to result in safer care.(22) It should also be noted that new safety technologies can introduce new errors.(23)
‘Smart pumps’ are considered not just in terms of workload of the staff that use the pumps but also in terms of the workflow and workload for pharmacists and other staff whose jobs may change as a result of implementing DERS.(14)
It is estimated that many hospitals in the UK have purchased ‘smart pumps’ but only relatively few sites have established drug libraries with associated dose limits. Before establishing drug libraries in infusion pumps, the user must first standardise the drug concentrations and dosing methods across the clinical profile areas within their hospital. This task is probably the main barrier to introducing a DERS.(15) Developing a drug library can be a daunting and time-consuming task, taking between 60 and 90 days,(24) requiring a coordinated project team of nursing, pharmacy and medical staff.
Because of the difficulties inherent in applying standardised concentrations to chemotherapy drugs, many oncology drugs will often either be left out of the drug library, or have wide dosing limits applied.(25)
Another major issue and drawback is that, without wireless connectivity, each pump must be physically located to update drug libraries and download log events. A number of sites without wireless implementations choose not to perform either regular event downloads or library updates because of the workload implications for clinical engineering staff.(14,26)
Factors influencing successful implementations
The introduction of a DERS is not straightforward and requires a focussed approach to facilitate a smooth implementation.(16) Some of the factors contributing to successful implementation include:
- A committed and representative stakeholder group (project team) within the hospital. Membership should include doctors, pharmacists, nurses, biomedical engineers and governance representatives.
- Ongoing support from the ‘smart pump’ manufacturer/supplier including pre-implementation education, supplier support and implementation guidance (consultancy), clinical training, post-implementation support, for example, infusion pump data downloading, uploading and reporting.
- A clear implementation plan that covers dataset development utilising agreed standard drug concentrations, targeted clinical profile areas, agreed safety limits, clinical training, implementation management/date, data review, analysis, learning and feedback plan.
Proposed drug templates
It is clear that developing and using standard drug concentrations can be a challenge when introducing ‘smart pumps’ with DERS. In addition, the availability and involvement of clinical pharmacists in this process can be problematic.(27) It is also accepted that the use of ‘nationally’ agreed drug templates to assist in the implementation of the DERS would be helpful.
The purpose of this paper is to attempt to provide a starting point for stakeholders when developing their drug template. The process is initiated for two areas where ‘smart pumps’ are commonly used: critical care (adult) and general medical/surgical.
The feasibility of achieving nationally agreed drug library templates will always prove problematic because of the large range of clinical treatments that may be required to be covered by DERS and the associated clinical opinion around this. In many ways it is surprising just to what extent intravenous medication practice differs between comparable centres. Even across the UK NHS, where one might expect consistency, studies have shown significant variations in practice.(28) Despite EU initiatives, there is still not even consistency in drug nomenclature across member nations and unfortunately, therefore, the production of internationally agreed drug library templates is at the moment unrealistic.
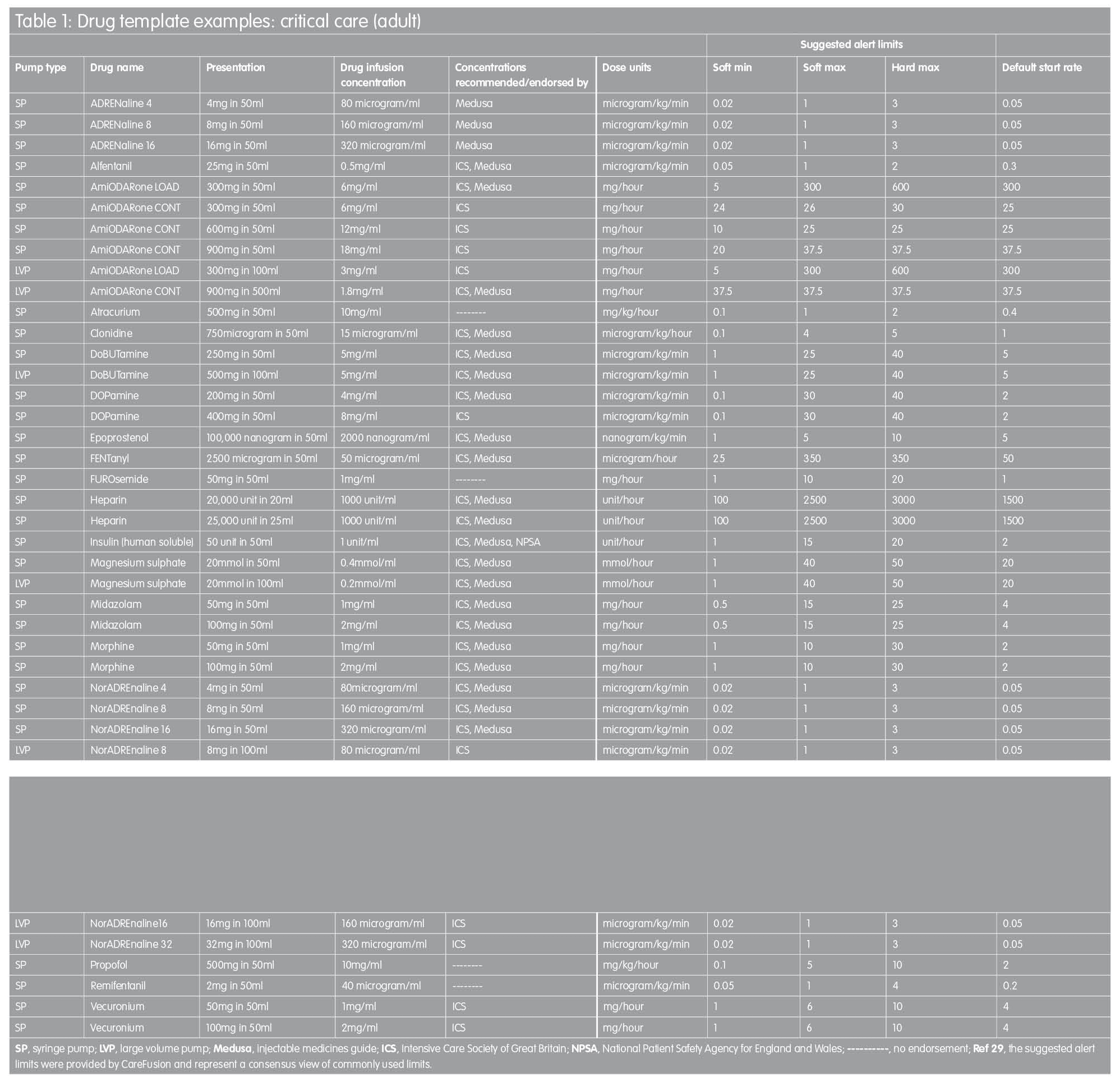
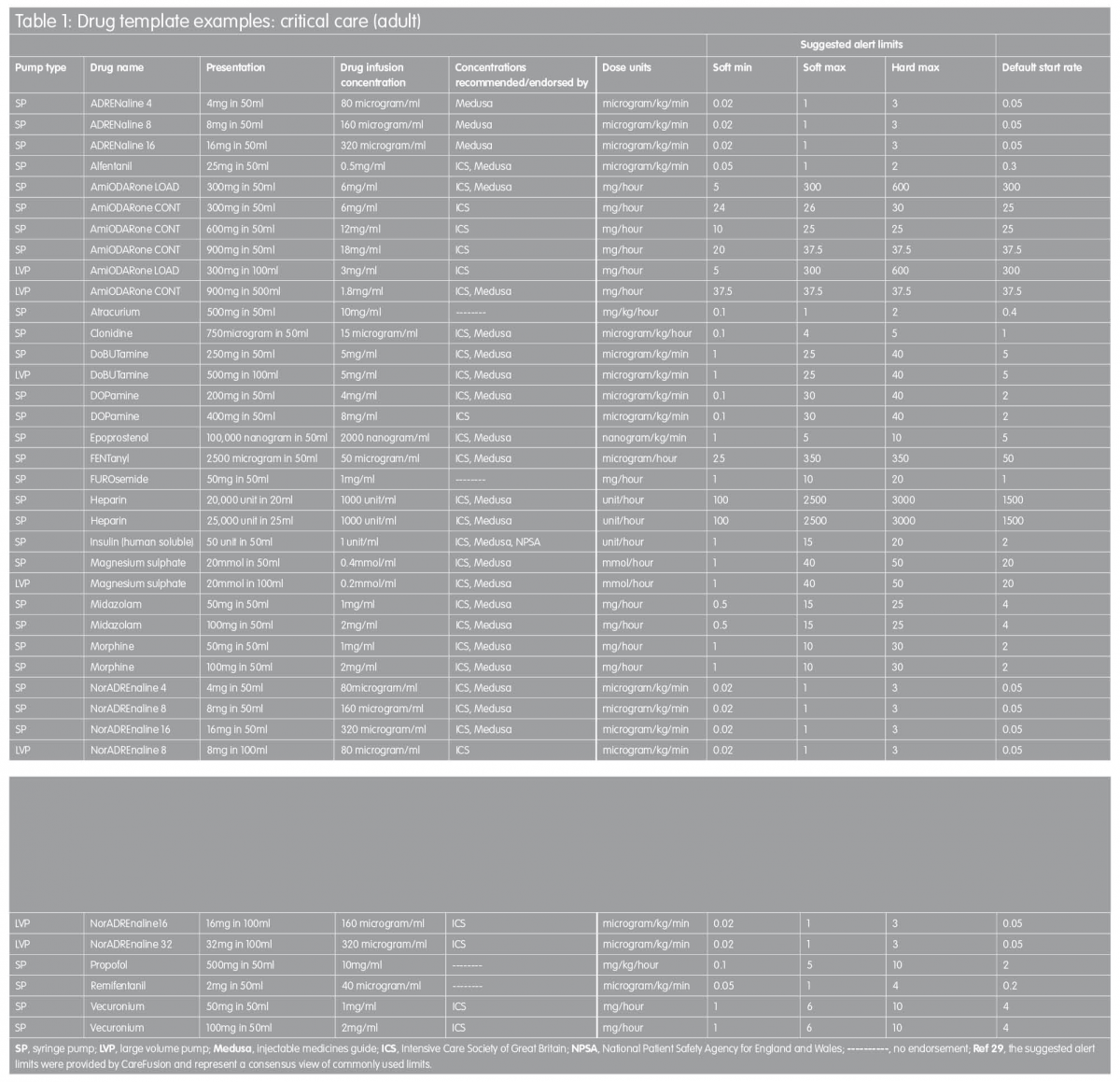
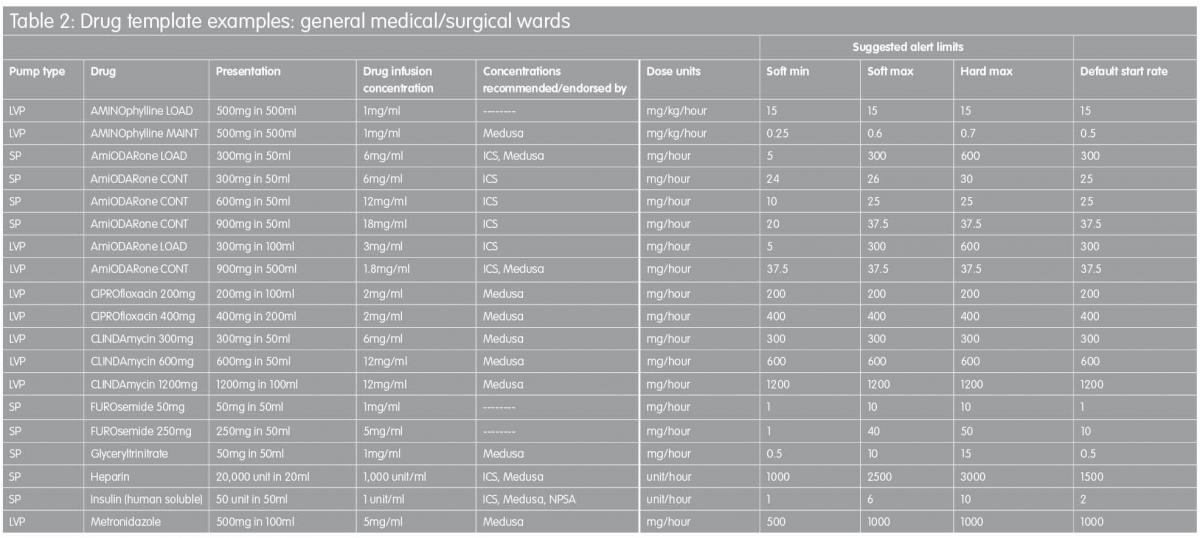
Proposed drug library templates for the UK
In order to facilitate the implementation of ‘smart pump’ technology, the templates in Tables 1 and 2 are proposed as a starting point for the development of individual hospitals’ drug library datasets. They are based on recommended standardised concentrations wherever possible, with infusion rate limits derived from a consensus of current practice established by customers of one infusion devices manufacturer across the UK.(29) In some instances, intravenous infusion of a named drug may not be a licensed use of that drug. In addition, standardised concentrations stipulated might be at variance with dilution directions quoted in the manufacturer’s Summary of Product Characteristics data. TaLLman lettering has been used throughout and is recommended as a means of reducing selection of the wrong drug from the drug library display on the infusion pump.
These drug templates are in no way intended to be prescriptive but they are intended to provide a starting point for those faced with the challenge of drug library development; the templates are designed to be edited by the local user to reflect accurately actual clinical practice. It is hoped to expand this work in the future in order to describe template drug libraries for other clinical areas, including specialist units such as neonatal intensive care, maternity, renal and oncology.
References
- Kohn LT, Corrigan JM, Donaldson MS. To Err is Human: Building a Safer Health Care System. Washington, DC: Institute of Medicine;1999.
- UK National Patient Safety Agency. Patient Safety Alert 20: Promoting safer use of injectable medicines. London:NPSA;2007.
- Wirtz V, Taxis K, Barbour ND. An observational study of intravenous medication errors in the United Kingdom and in Germany. Pharm World Sci 2003;25(3):104–11.
- Cousins DH et al. Medication errors in intravenous drug preparation and administration: a multicentre audit in the UK, Germany and France. Qual Saf Health Care 2005;14(3):190–5.
- McDowell SE et al. Where errors occur in the preparation and administration of intravenous medicines: A systematic review and Bayesian analysis. Qual Saf Health Care 2010;19(4):341–5.
- Westbrook J et al. Errors in the administration of intravenous medications in hospital and the role of correct procedures and nurse experience. BMJ Quality Saf 2011;20(12):1027–34.
- Council of Europe. Survey on medication errors. Strasbourg:Council of Europe;2003.
- Quinn C, Glenister H, Stephenson E. NPSA infusion device toolkit: a cost saving way to improve patient safety. CGIJ 2004;9:195–9.
- Snodgrass R. The fundamentals of smart pump technology. Biomed Instrument Technol 2005;39:444–6.
- National Patient Safety Agency. Patient safety Alert 20: Promoting safer use of injectable medicines. London:NPSA;2007.
- Borthwick M et al. Towards standardisation of drug infusion concentrations in UK critical care units. The Intensive Care Society;2009.
- Royal College of Nursing. Standards for Infusion Therapy 3rd Edition. RCN;2010.
- Hatcher I et al. An intravenous medication safety system. JONA 2004;34:437–9.
- Purchasing and Supply Agency. Dose Error Reduction Systems for Infusion Pumps: CEP 08034. London:NHS Purchasing & Supply Agency;2008.
- Murdoch LJ, Cameron VL. Smart infusion technology: a minimum safety standard for intensive care? Br J Nurs 2008;17(10):630–6.
- Quinn, C. Smart practice: the introduction of a dose error reduction system. Br J Nurs 2011;8(suppl):S20–S25.
- Medusa. Injectable Medicines Guide. [Online] 2012. www.injguide.nhs.uk.
- ICS GB. Medication concentrations in critical care areas. Intensive Care Society of Great Britain [Online] 2009. www.ics.ac.uk/intensive_care_professional/concentration_guidance.
- Parshuram CS et al. Systematic evaluation of errors occurring during the preparation of intravenous medication. Can Med Assoc J 2008;178:142–8.
- Institute for Safe Medication Practice (ISMP). Standard concentrations of neonatal drug infusions. [Online] 2011. www.ismp.org/Tools/PaediatricConcentrations.
- Dmondson A, Bohmer RM, Pisano G. Disrupted routines: Team learning and new technology implementation in hospitals. Admin Sci Quart 2001;46:686–716.
- Rothschild JM et al. A controlled trial of smart infusion pumps to improve medication safety in critically ill patients. Crit Care Med 2005;33(3):533–40.
- Nucklos TK et al. Programmable infusion devices in ICUs: An analysis of corresponding adverse drug events. J Gen Internal Med 2008;23:41–5.
- Disconsiglio J. Getting smart about safe infusion pump options. Materials Manage Healthcare 2005;14(10):26–30.
- ECRI. Get the facts on acquiring smarter pumps. ECRI, Plymouth, USA;2007.
- Hess J, Whiting S. Making an intelligent smart pump purchase. Materials Manage Healthcare 2007;16(8):38–41.
- Upton D. Why are so few infusions “smart”? Hosp Pharm Eur 2011;57:39–42.
- Borthwick M et al. Standardisation of drug infusion concentrations in the United Kingdom. JICS 2007;8:92–6.
- CareFusion. Personal communication with Mr Chris Quinn, Clinical Manager, CareFusion International.