teaser
B Roger Allen
MB FRCP
Consultant Dermatologist
Nottingham
UK
E:[email protected]
Tacrolimus ointment was first shown to be effective in the treatment of atopic dermatitis (AD) in a small proof-of-concept study published in 1994.(1) Since then, trials including over 16,000 patients have been reported and over three million patients worldwide have been treated. The efficacy of the drug is equivalent to a medium-potency topical steroid but with a more limited side-effect profile.
Despite theoretical concerns over safety, no serious side-effects have been confirmed, with application site burning, usually transient, being the only commonly reported adverse reaction. The drug therefore offers a safe and effective alternative to topical steroids in many patients with AD.
Atopic dermatitis
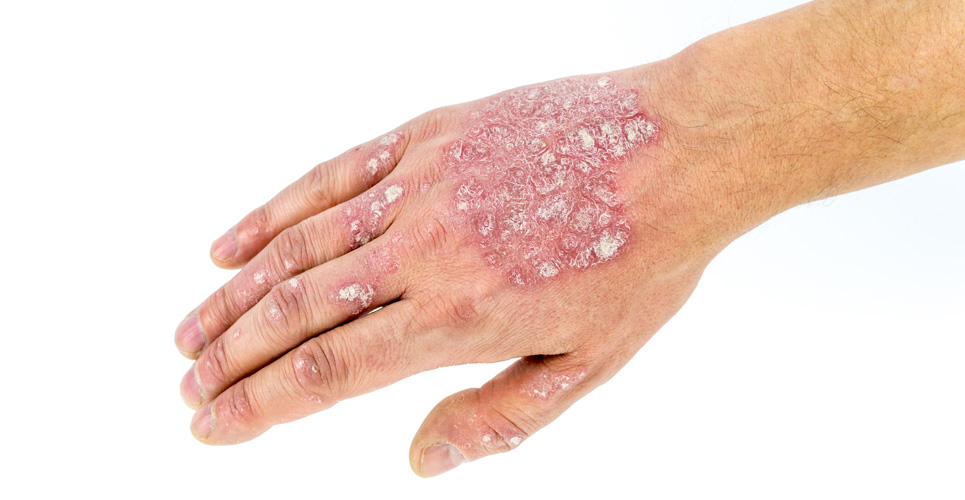
AD (see Figure 1) is the most common inflammatory skin disease of childhood. In developed countries, up to 20% of children are affected at some stage. The morbidity caused by the intense itching, which is the hallmark of the condition, is distressing for the patient and highly disruptive for the whole family.(2) The distress caused by AD has probably been underestimated, and a recent survey of over 2,000 patients has shown that, each year, patients spend one out of three days in flare. Concerns about therapy resulted in a delay of nearly a week before active treatment was initiated.(3)
[[HPE29_fig1_66]]
Since 1952,(4) cortico�’steroids have been the mainstay of topical treatment for AD. Modifications to the molecular structure have resulted in a range of highly effective preparations, but the more potent the corticosteroid, the greater the possibility of side-effects.
Until the introduction of tacrolimus (and pimecrolimus, which has a similar structure), no pharmacologically new compounds with proven efficacy in AD outside the class of corticosteroids have been available.
Discussion
Tacrolimus was first isolated in 1984(5) from a fungus-like bacterium, Streptomyces tsukubaensis, isolated from soil samples found on mount Tsukuba in Japan. Its immunosupressant properties were described in 1987.(6) The name is derived from Tsukuba macrolide immunosuppressant. Its mode of action is by inhibiting T-cell activation through inhibition of the enzyme calcineurin. This is similar to the action of ciclosporin and, in the transplantation field, it is used systemically as an alternative. However, unlike ciclosporin, tacrolimus exhibits activity when applied topically.(7)
Tacrolimus ointment was first shown to be effective in the treatment of AD in a small proof-of-concept study published in 1994.(1) As a result, tacrolimus in an ointment base has been developed specifically for the treatment of AD. It is the first in the new class of topical calcineurin inhibitors, and the targeted mode of action of these agents is likely to make them much more specific than any of the topical corticosteroids. The clinical development programme for tacrolimus ointment has been the biggest ever in the field of dermatology and has included more than 16,000 patients. It is now marketed as Protopic(R) 0.03% and 0.1% ointment and licensed as second-line therapy for the treatment of AD.
Key studies have included a 12-week study of efficacy in 630 adult patients using 0.03 and 0.1% tacrolimus. Both were significantly better than placebo,(8) with no serious safety issues, although approximately 40-55% of patients reported some skin burning.(9)
A comparable study in 351 children gave similar results(10) but with less trouble from burning.
In a comparative three-week study, 0.03% and 0.1% tacrolimus ointment were compared with 0.1% hydrocortisone butyrate ointment in the treatment of 570 adult patients with moderate-to-severe AD.(11) Tacrolimus was shown to have an efficacy similar to that of the steroid preparation. Transient local irritation in the tacrolimus treatment groups, particularly during the first few days, was again the most common adverse event. No serious safety concerns were identified.
Similarly, a study in 560 children showed 0.03% and 0.1% tacrolimus to be significantly more effective than 1% hydrocortisone acetate and a clear trend for 0.1% tacrolimus to be more effective than 0.03% tacrolimus.(12)
A more recent study in adults, which included just under 1,000 patients, showed that, over a six-month period, 0.1% tacrolimus was superior to 1.0% hydrocortisone acetate ointment on the face and 0.1% hydrocortisone butyrate on the body.(13)
Topical corticosteroids are very effective in the short-term treatment of AD. However, due to their well-documented local side-effects when used for prolonged periods of time (particularly skin atrophy and striae), long-term studies with continuous use of topical corticosteroids are not regarded as ethical and so no study has been conducted. The intermittent use of potent corticosteroids may improve the safety profile, but this has not been demonstrated. Amongst patients, concern over their use, and in particular concerning atrophy, is widespread.(14) Tacrolimus does not cause skin atrophy.(15)
Although this new class of topical calcineurin inhibitor is not licensed for maintenance therapy, there is the possibility of treating patients for prolonged periods of time or on repeated occasions, without the fear of the cumulative damage, such as skin atrophy, corticosteroid dermatoses or telangiectasia, associated with corticosteroids.
A long-term (one-year), single-arm study in adult patients showed an increasing percentage of patients categorised as having marked improvement or clearance over a one-year period.(16) While the percentage of patients in those categories after the first few weeks of treatment was not different from those seen in the short-term trials, a steadily increasing number of patients reached those categories on longer follow-up (54%, 81% and 86% of patients at week 1, month 6 and month 12, respectively). A rebound effect after stopping tacrolimus therapy was not seen. In this study, patients applied 0.1% tacrolimus ointment until seven days after improvement of the lesions and then restarted therapy whenever new lesions occurred.
Similarly, a long-term paediatric safety study in the USA showed no indication that the effectiveness of tacrolimus ointment decreases over time.(17) Improvements in disease status, as demonstrated by reductions in Eczema Area and Severity Index scores, percent body surface area affected, individual signs and symptoms of AD, and the patient’s assessment of pruritus, generally occurred during the first week of treatment and were maintained throughout the study.
Safety
It is rare for a completely new treatment to arrive on the scene, and when it does there is natural apprehension that all may not be well, or as it seems, but clinical trials and postmarketing surveillance have failed to reveal any safety issues with the use of topical tacrolimus.
Questions over potential long-term side-effects are bound to arise, although it may not be possible to answer some these for many decades.
The systemic absorption of tacrolimus is low;(16,18) consequently, any systemic immunosuppression seems unlikely. In a one-year follow-up study,(16) immunocompetence, as measured by the Recall Antigen Test, which has been used to assess delayed-type hypersensitivity reactions as an indirect measure of cellular immune response (Multitest(R) CMI, Institut Merieux, Lyon, France), showed a patient population with depressed cell-mediated immunity at baseline, but with no notable changes following prolonged treatment.
The incidence of skin infections does not appear to be increased.(19,20) The use of topical corticosteroids on the face, particularly around the eyes, must be restricted, as glaucoma can result, but localisation of AD to the eyelids is common (see Figure 2). An open-label trial of tacrolimus ointment in 20 patients with eyelid dermatitis showed no increase in intraocular pressures, which provides support for its safety in treating this area.(21)
[[HPE29_fig2_67]]
The issue of ultraviolet (UV) exposure has also been raised. A clinical study conducted with healthy human volunteers found no evidence of phototoxicity using tacrolimus ointment at concentrations up to 0.3%.(22) However, as a precaution, patients are advised to protect treated areas of skin from �concomitant sunlight or other UV light sources.
In view of the immunosuppressant nature of the drug, there is heightened awareness of a theoretical risk of increased malignancy, but, as indicated above, systemic exposure is low. Importantly, up to three years of therapy with tacrolimus ointment has so far failed to reveal any signs of increased risk of skin malignancies.(23,24) Out of a total of 9,813 patients, 1,718 person years of tacrolimus exposure accrued with adults ≥40 years of age. Thirteen patients (median age 61 years) were diagnosed with nonmelanoma skin cancer. No cases were considered related to treatment, and the figures are in agreement with a comparable population without AD. More experience will clearly be required to address this issue conclusively.
There have been reports of lymphoma and cutaneous malignancies in patients who have been treated with tacrolimus, and these have been reviewed by the EMEA, which decided that it was unable to conclude whether there was a link with treatment. It also concluded that the benefit outweighed the risks but that awareness of these potential risks should be raised for patents and prescribers.(25)
Conclusions
It is clear that tacrolimus ointment constitutes a major new development in the treatment of atopic dermatitis. Short- and long-term trials have demonstrated a positive risk/benefit ratio.
The safety profile of tacrolimus ointment indicates that these patients have not been exposed to any new or significant adverse effects, although, as with the introduction of any novel agent, we should remain alert to the possibility that long-term use in large numbers of patients could yet reveal hazards not identified in the extensive trials and postmarketing surveillance.
Tacrolimus ointment introduces the potential for long-term intermittent therapy in the treatment of moderate-to-severe AD, without the use of steroids, and its availability is likely to change the approaches taken to managing the disease.
References
- Lancet 1994;344:883.
- J Am Acad Dermatol 2001;45:S64-6.
- J Allergy Clin Immunol 2006;118:226-32.
- J Invest Dermatol 1952;19:101-2.
- Transplant Proc 1991;23:2713-7.
- J Antibiot (Tokyo) 1987;XL:1256-65.
- Lancet 1992;339:1120.
- J Am Acad Dermatol 2001;44:S28-38.
- J Am Acad Dermatol 2001;44:S39-46.
- J Am Acad Dermatol 2001;44:S47-57.
- J Allergy Clin Immunol 2002;109:547-55
- J Allergy Clin Immunol 2002;109:539-46
- Br J Dermatol 2005;152:1282-9.
- Br J Dermatol 2000;142:931-6.
- Br J Dermatol 2004;150:1174-81.
- Arch Dermatol 2000;136:999-1006.
- J Am Acad Dermatol 2001;44:S58-64.
- J Invest Dermatol 2005;124:695-9.
- J Allergy Clin Immunol 2001;107:196-7.
- J Am Acad Dermatol 2002;47:562-70.
- J Invest Dermatol 2001;117(2):533 Abstract 861.
- J Am Acad Dermatol 2001;44:S17-27.
- Arch Dermatol 2000;136;179-84.
- J Am Acad Dermatol 2005;53;S195-205.
- Doc Ref EMEA/98882/2006.
Resources
National Eczema Society
W:www.eczema.org
Astellas
W:www.astellas-europe.co.uk
Under My Skin
W:www.undermyskin.co.uk
European Medicines Evaluation Agency
W:www.emea.eu.int
British Association of Dermatologists
W:www.bad.org.uk