TiGenix NV, the European leader in cell therapy, has announced that it will develop its intravenous-administered allogeneic stem cell product, Cx611.
The product is for patients suffering from early rheumatoid arthritis (RA) and for patients suffering from severe sepsis, a potentially life-threatening complication of infection.
TiGenix NV, the European leader in cell therapy, has announced that it will develop its intravenous-administered allogeneic stem cell product, Cx611.
The product is for patients suffering from early rheumatoid arthritis (RA) and for patients suffering from severe sepsis, a potentially life-threatening complication of infection.
“Having considered the demonstrated therapeutic effects of allogeneic stem cells, the animal and clinical data for Cx611 collected so far, the potential applications into areas of high unmet medical need, and the advice from clinical experts in Europe and in the United States, we are convinced that it is in early rheumatoid arthritis and in severe sepsis that we should concentrate our resources for Cx611” said Eduardo Bravo, CEO of TiGenix. “In early rheumatoid arthritis, Cx611 could offer patients a therapy with an alternative mechanism of action that delays the need to progress to biological drugs. In severe sepsis, Cx611 could be a therapy with a mechanism of action that provides significant advantages when combined with normal standards of care, delivering faster recovery and improved survival rates. Success in any of these indications would represent a major medical and commercial opportunity for Cx611.”
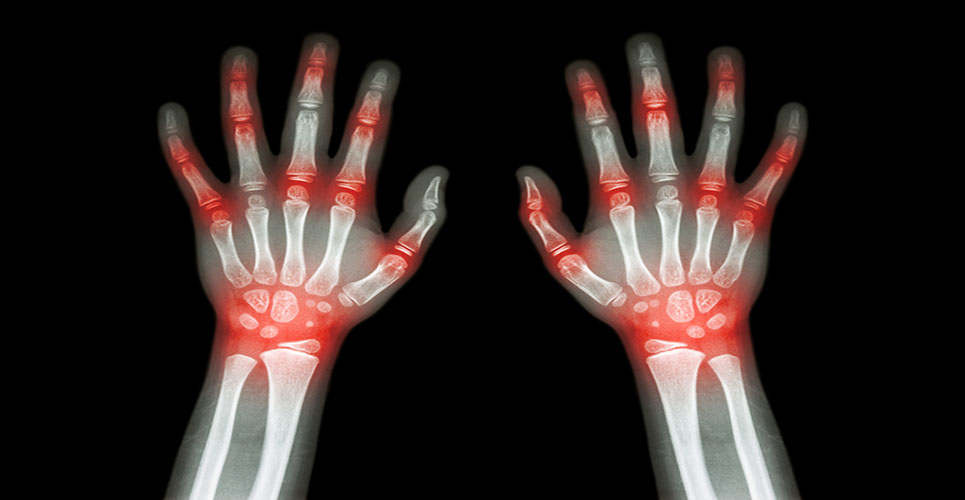
Early rheumatoid arthritis
RA is a chronic polyarticular inflammatory joint disease typically involving the small joints of the hands and feet that affects between 0.5 and 1% of adults in the developed world. Between 5 and 50 per 100,000 people develop the condition each year. After initial treatment with methotrexate and non-steroidal anti-inflammatory drugs (NSAIDs), and/or corticosteroids, disease activity in a number of patients is insufficiently controlled and typically leads to treatment with a group of drugs known as ‘biologicals’, such as the TNF-alpha inhibitors. Nevertheless, lack of an adequate response in a significant portion of treated patients, safety concerns and declining efficacy over time, requiring a substantial number of patients to periodically change drugs, indicate the need for additional therapeutic approaches.
“There is a need for a treatment with an alternative mechanism of action that could increase the proportion of patients brought into remission and reduce the need to progress to chronic, sequential and expensive biological therapies,” said Frank Luyten, Professor and Chairman of Rheumatology, University Hospital of Leuven, Belgium.
In animal models, expanded adipose-derived stem cells (eASCs) have been shown to down-regulate pro-inflammatory cytokines and up-regulate regulatory T cells which modulate the immune system. In a phase IIa study of Cx611 in refractory RA completed and presented in 2013, the safety of the product was confirmed and signs of efficacy were encouraging. Some patients treated with Cx611 entered remission after years of treatment with conventional and biological drugs.
Assisted by a Steering Committee consisting of Professor Mark Genovese (Professor Immunology and Rheumatology, Stanford University, US), Professor Paul Emery (Professor of Rheumatology, University of Leeds, UK) and Professor José María Alvaro-Gracia (Head of the Biological Therapies Unit at the Hospital Universitario de La Princesa, Madrid, Spain), the principal investigator in the earlier phase IIa study of Cx611 in refractory RA, TiGenix is working with a group of leading clinical experts, including Professor Luyten (Belgium), to complete the protocol for a randomised, double-blind, comparative phase II study to test the efficacy of Cx611 in patients exhibiting substantial disease activity of RA despite treatment with methotrexate and corticosteroids, but unexposed to a biological drug. Recruitment for the proposed study could start in the third quarter of 2015 and TiGenix would expect final results to be available by the first half of 2017.
Severe sepsis
Sepsis is a potentially life-threatening complication of infection. Sepsis occurs when inflammatory molecules released into the bloodstream to fight the infection trigger systemic inflammation. This inflammation can trigger a cascade of detrimental changes that damage multiple organ systems, causing them to fail. If sepsis progresses to septic shock, blood pressure drops dramatically, which may lead to death. People with severe sepsis require close monitoring and treatment in a hospital intensive care unit. Drug therapy is likely to include broad-spectrum antibiotics, corticosteroids, vasopressor drugs to increase blood pressure, as well as oxygen and large amounts of intravenous fluids. Supportive therapy may be needed to stabilise breathing and heart function and to replace kidney function.
“Even today, patients with severe sepsis have a low survival rate so there is a critical need to improve the effectiveness of current therapy,” said Professor Pierre-François Laterre, Professor of Medicine and Head of Intensive Care, Saint Luc University Hospital, Brussels, Belgium. “Only a small number of new molecular entities are currently in development for severe sepsis. Based on the available data, I believe there is a strong rationale to study Cx611 in this patient population.”
In animal models, eASCs have been shown both to decrease pro-inflammatory mediators and increase anti-inflammatory mediators, and to have anti-microbial effects. As a result of this mechanism of action, Cx611 has demonstrated efficacy in significantly reducing mortality in two animal models of sepsis. TiGenix therefore believes that Cx611 has a potentially important role when combined with current standards of care in patients with severe sepsis.
The company is working on the development plan of Cx611 in this indication with an Advisory Board of Professor Pierre-François Laterre, Dr Bruno François (Service de Réanimation Polyvalente, Centre Hospitalier Universitaire, Limoges, France), Professor Sébastien Gibot (Service de Réanimation Médicale, Centre Hospitalier Universitaire, Nancy, France) and Professor Tom van der Poll (Department of Internal Medicine, Academic Medical Center, University of Amsterdam, The Netherlands). As well as additional animal model testing, TiGenix will start a randomised, placebo-controlled trial to test the mechanism of action of Cx611 in healthy volunteers challenged with a bacterial endotoxin (lipopolysaccharide), a potent pro-inflammatory constituent of the outer membrane of Gram-negative bacteria, which elicits a strong inflammatory response inducing sepsis-like clinical symptoms. TiGenix expects to complete this study by the third quarter of 2015 and follow up with a phase II trial of Cx611 as an add-on therapy to the standard of care in patients with severe sepsis.