Parenteral nutrition (PN) is a mode of therapy established for patients who are intolerant of, or those who cannot receive adequate nutrition via the oral or enteral route. It is a complex nutritional admixture composed of many different chemical entities which must be chemically, physically and microbiologically robust in order to be safely administered to the patient.1 It is given to patients as either bespoke, manually compounded bags or pre-filled ‘standard’ bags, while they are in hospital or at home. Standard bags are also known as multi-chamber bags (MCB).
Indications for PN
The National Institute for Health and Care Excellence (NICE) has clearly defined candidates for PN as being those who are malnourished, or at risk of malnutrition, as well as being either unsafe for oral nutrition (for example, require gut rest) or having inaccessible or functional problems associated with the gastrointestinal (GI) tract.2 This includes acute and chronic conditions such as short bowel syndrome, malabsorption, inflammatory bowel disease, fistulae, obstruction and complications relating to GI surgery.3 While the enteral route would be the first choice, it can fail to provide sufficient nutrient intake in a number of patients, necessitating the use of PN.
PN is often referred to as ‘total’ PN: in this sense it aims to provide the complete nutritional needs of the patient without any significant enteral intake. However, PN is also given supplementary to nutrition consumed via the GI tract in patients who are still capable of oral or enteral feeding yet who cannot fully sustain themselves.4
PN production and administration
The basic formula for PN includes a mixture of lipid (as an emulsion), carbohydrate (as glucose solution), amino acid solution (including essential and non-essential amino acids), vitamins, trace elements, electrolytes and water, and can be produced as:
- ‘Bespoke’ bags (feeds): compounded from individual components as per individualised patient needs, usually for patients with sensitive and/or long-term requirements (outside of remit of standard bags)
- ‘Standard’ bags: pre-compounded pre-filled PN bags, which are licensed ready-to-use pharmaceutical products with a set formulation/composition. Produced for convenience to cater for more generalised nutritional needs, having longer shelf lives and no requirement for refrigeration. These can be given with or without subsequent additions of electrolytes and/or micronutrients (standard vs tailored regimen).
Standard PN feeds are usually supplied as industrially manufactured, terminally sterilised MCBs. The components are physically separated in pre-filled chambers to reduce physical or chemical instability or incompatibility. When required, the bags are manually rolled, applying external pressure to open the weak inner seals, mixing the chambers contents to form a uniform formulation. Triple-chamber (all-in-one) bags contain lipid, glucose and amino acid solutions, whereas the lipid component is omitted in the dual-chamber (two-in-one) bags, which may be more appropriate for some patients, for example, those with concomitant cholestatic liver disease or intestinal failure-associated liver disease (IFALD). For convenience, baseline electrolyte levels are included in many formulations to meet the general needs of most patients; however, electrolyte-free options are also available.
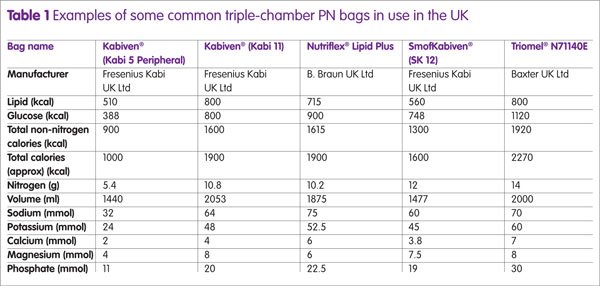
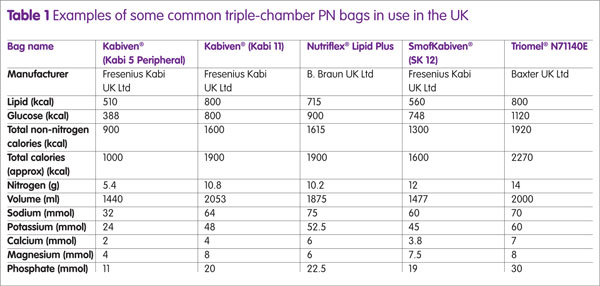
Additionally, some products are licensed for use in paediatrics and/or for peripheral administration. Table 1 gives examples of some common triple-chamber bags and their composition. Additional fluids, electrolytes, vitamins and TE may be added (up to limits set by manufacturer) via injection port after the PN has been mixed.5 The National Patient Safety Association (NPSA) recommends that all additions should be carried out with care and performed using aseptic best practices to limit the risk of error and contamination to the feed.6 The choice of commercially available PN formulations is continually growing, giving hospital pharmacies without compounding facilities the opportunity to provide the most suitable triple-chamber formulations to meet individual patient needs. Some PN formulations are now available with added micronutrients and extended shelf-lives, which can be stored in a refrigerator.
The advantages of using standard prefilled PN bags are that they avoid the need to compound bags locally, and avoid the costs associated with purchasing bespoke or aseptically prepared bags. Also, they do not require refrigeration, which makes them a useful option for stable home PN patients for short periods away (for example, for holidays). Bespoke bags require sterile aseptic preparation conditions and recently ASPEN has issued standardised competencies and safe practice recommendations regarding their order, review, preparation (including compounding) to reduce risks from difference in local procedures.7
Patient requirements
For patients who are still able to consume nutrition orally, the extent should be taken into account in the first instance to gauge estimated patient requirements; alongside other factors including knowledge of patients’ remaining gut anatomy, GI absorption, intended activity needs, underlying diseases and any fistula/stomal losses. As such, patient PN requirements vary considerably and current ‘standard’ bags aim to cater for a range of patient needs. For patients who are initially commenced on PN, their requirements are based on best estimates and then further refined over time according to the patients’ state of hydration and target weight as well as their personal preferences. Table 1 shows some examples of commercially available standard bags; in reality a greater variety is available for consumer use which differ in composition and ratios of both macronutrients and electrolytes. When choosing an MCB for an individual patient, the most suitable MCB should be cross-matched to the individual patient’s nutritional requirements.
In these instances, the final selection may depend on certain clinical considerations. For example, in the absence of the ‘perfect’ bag, a bag with a better volume and electrolyte profile, but with a minor shortfall in calories, may be preferential for a patient requiring short-term PN (days). It is important to recognise situations when MCBs may be unsuitable, such as in patients at risk of refeeding syndrome or those with extreme disease states (high output fistula, intestinal failure, liver disease).
Glucose and lipid
Glucose and lipid comprise the main sources of energy in PN, with recommended glucose requirements set at 3–6g/kg body weight/day.8 Most patients are provided with lipid, yet efforts are made to ensure its long-term provision is kept below 1g/kg body weight/day as over-provision is associated with chronic cholestasis and IFALD.8,9 By contrast, caution needs to be taken with glucose provision as excess causes hyperglycaemia and likewise liver damage (deranged liver function tests (LFTs), steatosis/‘fatty’ liver), in which case, calculation of glucose oxidation rate gives an idea of the maximum amount of glucose an individual’s body is able to utilise.10,11
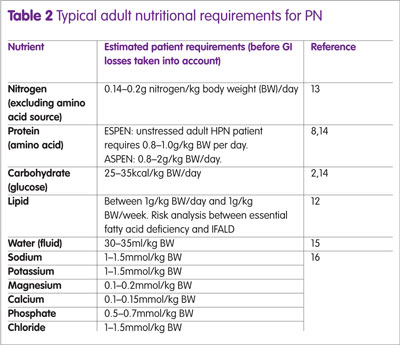
Initially, patients are given glucose and lipid at a ratio of 50:50 (or 60:40), as provided by most triple-chamber bags; in subsequent months this ratio is then reduced to 70:30 (or 85:15) for patients with long-term PN requirements.8 If patients show evidence of deranged liver function, for example, raised LFTs or cholestatic liver disease, then consideration should be given to reducing or stopping parenteral lipid provision. Table 2 gives guidelines for typical adult nutritional requirements (before GI losses are taken into account).
It is worth noting that the source of lipid in triple-chamber bags differ between manufacturers. Lipid emulsions containing LCT (for example, Intralipid®) or a mixture of LCT:MCT (for example, Lipofundin®) have proven established use in PN. Meanwhile, more recent olive oil (for example, SMOFLipid®, Clinoleic®) and structured lipid emulsions (for example, Structolipid®) have also been safely used in PN, yet this area still requires significant research to consolidate their preferential use.12
Micronutrient additions and requirements
Triple and dual MCBs are not pre-formulated with vitamins and trace elements, due to stability issues, so these may need to be added. NICE guidelines state that all patients should receive appropriate provision of micronutrients from the outset of feeding;2 however there is still a lack of consensus on supplementing patients’ micronutrient needs in acute illness. Some practitioners take the attitude that PN supplementation over short periods is unlikely to be clinically relevant, yet others have argued that increased oxidative stress during illness warrants increased provision, particularly for antioxidants (for example, vitamin E, selenium and zinc) which have roles in wound healing and immune function. Conversely, some micronutrients are commonly found in excess in patients, for example, manganese and copper, so it may not be appropriate for their extra provision. Unfortunately, micronutrients are most often provided as a combined preparation (for example, Cernevit®, Vitlipid N Adult®), which contain levels of micronutrients that may be inappropriate for specific patients. Recent research has shown that levels of manganese, selenium and vitamin D in such products may be unsuitable for the long-term needs of home PN patients.17
Overall, during the early stages of starting PN, clinical goals should focus on biochemical stabilisation with limited calorie provision (rather than aiming for weight gain) alongside adequate electrolyte and micronutrient provision.
Discussion
The advantages (prolonged shelf-life, low relative cost) of using MCBs do not always outweigh the negatives (less scope for manipulation of individual PN requirements): they are devoid of micronutrients and often contain less than adequate electrolytes, as the electrolyte content in MCBs are usually derived from the existing components of the bag rather than being designed to meet standard patient requirements. Without manual additions, most patients run the risk of deranged blood electrolyte levels/suboptimal electrolyte provision.
A retrospective study by Beattie et al compared bespoke compounded bags to premixed standard bags and found that provision of glucose, lipid and electrolytes differed greatly, yet came to the conclusion that premixed bags hold a role in quaternary centres for stable non-critically ill patients.18 However a recent multi-centre randomised trial found that the use of triple-chamber bags was comparable to traditional compounded bags as demonstrated by relative differences in pre-albumin and CRP values. The study did not report differences in length of stay, 30-day mortality or postoperative adverse events, demonstrating the safe, simplified and cost-effective use of MCBs over compounded bags.19 The British Pharmaceutical Nutrition Group (BPNG)issued a statement in 2010 detailing recommendations for the use of MCBs.
They acknowledged that MCBs have a place in clinical practice, but cited findings from their recent study that over half of the PN manufactured in the UK incorporated the use of MCBs, with 28% being given without specialist assessment and 33% being given without essential additives, ultimately demonstrating that MCBs are not being given to patients as clinically recommended and often with a ‘one size fits all’ attitude.20 Their recommendations emphasised that PN should only be commenced after consultation with the nutrition support team (NST), nutritional screening assessment, consideration of patient requirements and route of access. Also, that if only MCBs are available, the choice must match the patient requirements with other additions to be considered appropriately (whether to the bag itself or via an alternative route of administration). In terms of safe practice, they stressed that MCBs should not be kept as ward or emergency stock, nor additions made at ward level.
Conclusions
In reality, the perceived inherent advantages of MCBs are also responsible for their disadvantages. Their apparent convenience and intended ease of use can result in trusts not stocking a broad enough range of bags as well as a ‘one bag fits all’ attitude rather than more clinical consideration of patient requirements, for example, extra additions of fluids or electrolytes. Only those clinicians (members of the NST) with appropriate clinical experience and awareness of the issues surrounding their use should dictate the use of MCB PN bags. Nevertheless, MCBs still offer a convenient means to cater for the general and minimally variable needs of most patients requiring PN; yet their use still creates a difficult/out of practice environment in that we may be inadequately treating those requiring bespoke services with more abstract requirements, for example, high/low volumes, greater electrolyte or calorie needs; in which case, referral to more specialised centres is recommended. Trusts should ensure that adequate training and investment is given to the staff and facilities that provide PN services so that optimal service provision is given to patients. Also, that the most suitable multi-chamber PN bag is recommended in light of the individual patient requirements, taking into account/considering all the clinical parameters and eventualities.
In conclusion, safe optimal usage of triple-chamber bags is achieved with core education and training, alongside guidance from health care professionals experienced in their application in aseptic practices and intravenous nutrition. Guidance recommends the use of specialised NSTs to manage PN patients, long acknowledged to provide optimal and safest standard of care, as well as monitoring of PN treatment, promoting adherence, minimising complications.8,21
Key points
- Triple- and dual-chamber bags, also known as multi-chamber bags (MCB), offer a convenient, safe and stable means for delivery of parenteral nutrition (PN) to patients for typical day-to-day requirements.
- As licensed products, MCBs contain the three macro components of PN (lipid emulsion, glucose, amino acid solutions) separately stored in chambers prior to rolling and mixing.
- MCBs are available as a range of commercial products to meet the variable needs of patients requiring nutritive support.
- Healthcare professionals involved in the provision of PN to patients need to be aware of issues surrounding their safe and optimal use to meet patient needs.
- Their simplistic nature and intended ease of use can pose unintentional risk without adequate clinical consideration.
References
1 White R. Parenteral nutrition for adults – an overview of the basic principles. Clin Pharm 2011;3:183–4.
2 National Institute for Health and Care Excellence. CG32. Nutrition support in adults: Oral nutrition support, enteral tube feeding and parenteral nutrition. London;2006. www.nice.org.uk/guidance/cg32 (accessed June 2017).
3 Pironi L et al. ESPEN endorsed recommendations. Definition and classification of intestinal failure in adults. Clin Nutr 2015;34(2):171–80.
4 Pertkiewicz M et al. Basics in clinical nutrition: Composition of nutritional admixtures and formulas for parenteral nutrition. Clin Nutr Metab 2009;4(4):e161–3.
5 Barnett MI et al. Basics in clinical nutrition: Parenteral nutrition admixtures, how to prepare parenteral nutrition (PN) admixtures. Eur E J Clin Nutr Metab;2009(4(3):e114–6. http://linkinghub.elsevier.com/retrieve/pii/S1751499109000031 (accessed June 2017).
6 National Patient Safety Agency. Patient Safety Alert 20 Promoting safer use of injectable medicines. London;2007.
7 Boullata JI et al. Standardized competencies for parenteral nutrition. Order review and parenteral nutrition preparation, including compounding: The ASPEN Model. Nutr Clin Pr 2016;31(4):548–55.
8 Staun M et al. ESPEN Guidelines on Parenteral Nutrition: home parenteral nutrition (HPN) in adult patients. Clin Nutr 2009;28(4):467–79.
9 Cavicchi M et al. Prevalence of liver disease and contributing factors in patients receiving home parenteral nutrition for permanent intestinal failure. Ann Intern Med 2000;132:525–32.
10 Rye B, Nightingale J. Adult fluid and nutritional requirements for home parenteral nutrition. In: Bozzetti F, Staun M, Van Gossum A (eds) Home Parenteral Nutrition, 2nd edn. Oxford, UK: CAB International;2015:219–28.
11 Hartl WH et al. Complications and monitoring – Guidelines on parenteral nutrition (chapter 11). Ger Med Sci 2009;7:1–12.
12 Dupont B et al. Use of lipids in home parenteral nutrition. In: Home Parenteral Nutrition, 2nd edn. Oxford: CAB International; 2015. p. 239–51.
13 Elia M. Artificial nutritional support. Med Int 1990;82:3392–6.
14 ASPEN Board of Directors and the Guidelines Clinical Task Force. Guidelines for the use of parenteral and enteral nutrition in adult and pediatric patients. JPEN 2002;26(1):1SA–138SA.
15 Tyler DS. Fluid and electrolytes. 2nd Ed. Lyerly HK (ed). The Handbook of Surgical Intensive Care. Chicago, Illinois: Year Book Medical Publishers, Inc;1989:223–50.
16 Micklewright A, Todorovic V. A Pocket Guide to Clinical Nutrition;1997.
17 Dodington S et al. Nutritional abnormalities in long-term parenteral nutrition patients. IJPP 2016;(S3):96.
18 Beattie C, Allard J, Raman M. Comparison between premixed and compounded parenteral nutrition solutions in hospitalized patients requiring parenteral nutrition. Nutr Clin Pr 2016;31(2):229–34.
19 Jianchun Y et al. Efficacy, safety, and preparation of standardized parenteral nutrition regimens: Three-chamber bags vs compounded monobags –
A prospective, multicenter, randomized, single-blind clinical trial. Nutr Clin Pr 2017;884533617701883 [Epub ahead of print].
20 British Pharmaceutical Nutrition Group. Position statement on the use of multi-chamber parenteral nutrition bags for use in adult patients;2010.
21 Parent B et al. Parenteral nutrition utilization after implementation of multidisciplinary nutrition support team oversight: A prospective cohort study. JPEN 2016;40(8):1151–7.