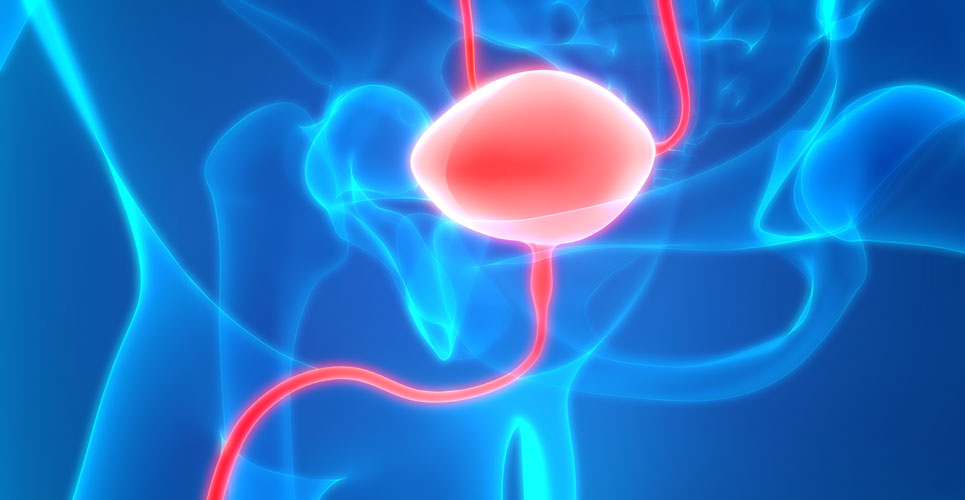
UCB drug Toviaz (fesoterodine fumarate), which has already been approved for use in the EU, has now gained approval from the US FDA for the treatment of overactive bladder with symptoms of urge urinary incontinence, urgency and urinary frequency.
The NDA approval was granted to Pfizer Inc who, in April 2006, acquired the exclusive worldwide rights to Toviaz from Schwarz Pharma.
UCB will be entitled to receive royalties on the combined sales of Toviaz and Pfizer’s current Detrol (tolterodine) product line and upon approval, the pharmaceutical giant will also receive a milestone payment from Pfizer, which is yet to be disclosed.
Toviaz is structurally related to Pfizer’s overactive bladder medication Detrol LA (tolterodine tartrate extended-release capsules).
The 4mg and 8 mg doses of Toviaz allow dosing flexibility to optimise treatment based on individual patient response and tolerability.