teaser
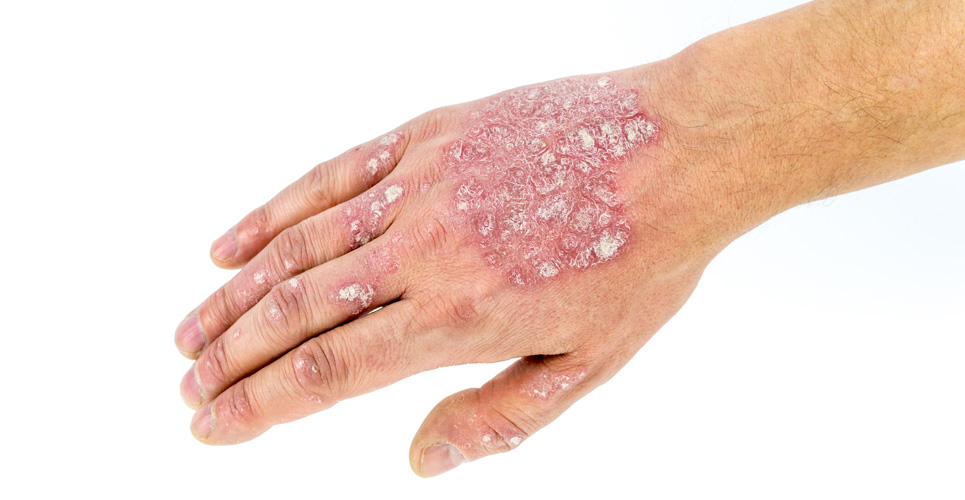
The National Institute for Health and Clinical Excellence (NICE) has issued draft guidance on the use of infliximab for the treatment of psoriasis within the NHS in England and Wales.
The main recommendations of this Final Appraisal Determination (FAD) are as follows:
1) Infliximab, within its licensed indications, is recommended as a treatment option for adults with plaque psoriasis only when the following criteria are met:
* The disease is very severe as defined by a total Psoriasis Area Severity Index (PASI) of 20 or more and a Dermatology Life Quality Index (DLQI) of more than 18;
* The psoriasis has failed to respond to standard systemic therapies such as ciclosporin, methotrexate or PUVA (psoralen and long-wave ultraviolet radiation), or the person is intolerant to or has a contraindication to these treatments.
2) Infliximab treatment should be continued beyond 10 weeks only in people whose psoriasis has shown an adequate response to treatment within 10 weeks. An adequate response is defined as either i) a 75% reduction in the PASI score from when treatment started (PASI 75) or ii) a 50% reduction in the PASI score (PASI 50) and a five-point reduction in the DLQI from when treatment started.
Health care professionals are advised to ensure that they take account of a patient’s disabilities or linguistic or other communication difficulties, when using the DLQI to reach conclusions on the severity of plaque psoriasis (the same applies to decision making in terms of deciding whether an adequate response has been achieved).
Formal consultees for this guidance have until 13 December 2007 to make any appeals.