Historically, pregnancy was thought to be protective against psychiatric illness, but there is a high rate of relapse in patients with existing mental health conditions who stop their psychotropic medication. Estimates of relapse vary but are 50% of patients with schizophrenia, 68% of patients with major depression, and upwards of 80% patients with bipolar disorder. In bipolar disorder, the relapse rates reduce to 29–37% in those who continue their medication.1 The Bipolar Disorder Research Network (BDRN) website (http://bdrn.org/) has some useful information on risks associated with a bipolar diagnosis and notes factors, such as poor sleep, as triggers for a manic episode. Women with a previous history of depression, a family history of a mood disorder, or depression during the current pregnancy are at increased risk for postpartum depression.2
If you choose to treat with medication, then the aim should be full resolution of symptoms, even if it means increasing the dose, as otherwise the foetus is exposed to both medication and illness.
Planning a pregnancy
There are many factors that can make for a healthy pregnancy and although the primary focus of this article is on psychotropic medications, a broader holistic assessment of the patient’s circumstances is required. If the patient already has a psychiatric diagnosis, this can impact on risk of relapse. Second pregnancies may carry different levels of risk but, from the patient’s perspective, what happened before is likely to influence some of their decisions.
If a patient’s pre-existing mental health condition has a consistent cyclical element, then aiming to conceive when at lower risk of a breakthrough episode may be considered, although, in practice, this may be difficult to achieve. The preconception stage is a good place to look at wider nutritional needs, some of which may be linked to diet and lifestyle.3 Key points to note are:
- Hazards of smoking, alcohol and use of illicit substances
- Alcohol is a known teratogen and when consumed during pregnancy may impair development of the foetal nervous system. There is no recommended minimum safe level and chronic ingestion of more than 5 units/day is a likely predictor for foetal alcohol syndrome.
- Any exposure to tobacco smoke has been linked to foetal malformations and morbidity and later neurodevelopmental problems in the infant, so a reminder about passive smoking may be helpful. Nicotine replacement therapy is preferable to sourcing nicotine via smoking but nicotine itself is a vasoconstrictor and can reduce placental perfusion and contribute to low birth weight seen in pregnant smokers.4
- The value of folic acid supplementation, noting that 5mg doses should be routinely given to women who are obese (body mass index >30kg/m2)
- Higher vitamin D supplements (1000IU daily) are also indicated in obese women, and vitamin D deficiency should be suspected in women who do not get adequate exposure to sunlight. These patients may require up to 20,000IU weekly for four to six weeks.
- Iron (30mg per day) and calcium (1500–2000mg per day) supplementation
With regard to existing psychotropic treatment, the weight of diagnosis as a risk factor for relapse must be accommodated. Information to support risk assessment of existing treatment during pregnancy sits within many resources, including generic sources such as the British National Formulary, as well as more specialised websites (see resources). Most detail is likely to be found at TOXBASE (registration required), which is part of the UK Teratology Information Service (UKTIS). Linked to UKTIS is a very user-friendly website called BUMPS. This site is patient-oriented and houses good quality information leaflets; the site also encourages patients to register their pregnancy outcomes and thus help grow the database of knowledge of pregnancy outcomes with medicines. Hence, reviewing treatment, optimising dose, and switching to alternative treatments that carry lower risk of foetal toxicity if appropriate can be undertaken with reference to a number of resources to support you in discussions with the patient and partner. Healthcare professionals must not forget the yellow card system if any untoward events do occur with prescribed treatments.
Suddenly I’m pregnant
The key message is that an unwell mother is a risk factor for a poor outcome. If good psychological wellbeing involves continuing with psychotropic agents, then that should be the default starting point.
Do not panic and stop treatment abruptly. Even psychotropics that do not carry any recognised acute discontinuation symptoms (for example, lithium) carry a high risk of relapse if discontinued abruptly, especially when lithium is used in the treatment of bipolar disorder. At this stage the patient is likely to be well in to the first trimester and drugs that have particular first trimester issues may well have done any damage if indeed they were ever going to. So abrupt cessation may offer no advantage. Many scans and tests can now be done to check on potential foetal harm and your obstetric department may have links and networks with mental health services which can facilitate joint working. If not, pharmacy personnel working in the respective services can informally network to optimise patient care. Specific information on psychotropic medication covering use during pregnancy and lactation can be found on the websites listed under resources. The local specialist mental health pharmacist is able to provide more details of local arrangements for accessing the choice and medication website. Many patients may have already done internet research of their current meds, spoken to other healthcare professionals and, depending on these and other influences, may need time to work through their options. Allow for the patient changing her mind. Shared decision making is key.
Initiating psychotropics during pregnancy
Some mental health conditions may present for the first time during the perinatal period and depression is a good example, so your involvement may be to help select a suitable antidepressant or other psychotropic agent. More specific information and nuances within the various drug groups are provided later in the article and summarised in Table 1. The National Institute for Health and Care Excellence (NICE) guidelines on antenatal and perinatal mental health provide treatment pathways for pharmacological selection and SSRIs are realistically the first line option in depression. For patients who want to minimise medication use it may be possible to trade some antidepressant pharmacology for high intensive psychological intervention (this is usually cognitive behavioural therapy). Any relapse is likely to require medication but consider electroconvulsive therapy not only for severe depression but also for mania and catatonia.5
It is important to review drug doses because secondary pharmacokinetic changes occur during pregnancy. These include increased volume of distribution, increased renal blood flow leading to increased glomerular filtration rate and drug elimination. With liver enzymes, CYP3A4 and CYP2D6 activity is increased while CYP1A2 activity is decreased.6 For drugs with a narrow therapeutic index or heavily reliant on one particular cytochrome enzyme for metabolism, then dose adjustment may be required. With respect to lithium, a dose increase may be required during the second and third trimesters due to increased volume of distribution.
Specific drug groups
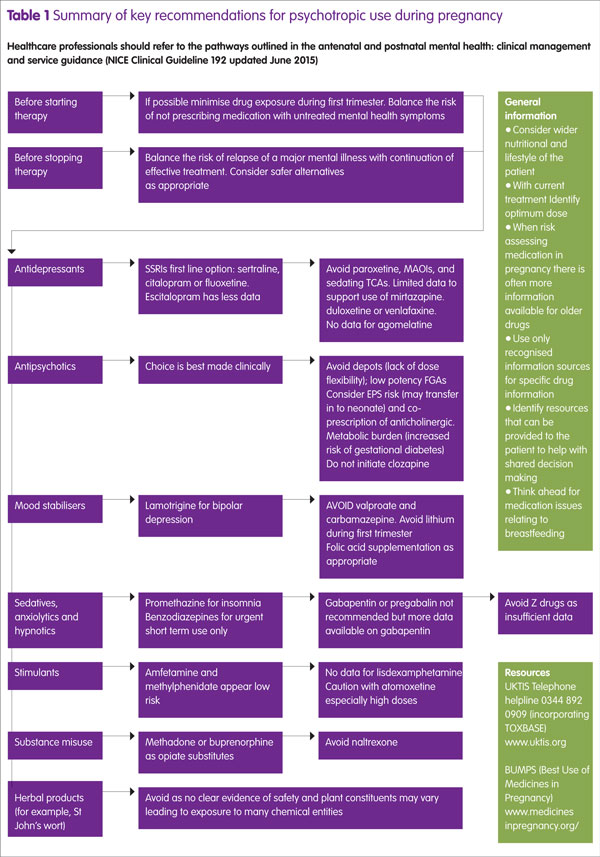
Traditionally the psychotropic drugs considered ‘red flag’ for use during pregnancy are valproate, carbamazepine, lithium, clozapine and benzodiazepines, but even they should not be seen automatically as absolute contra-indications if the mother’s positive mental health cannot be achieved without them.

Mood stabilisers
Valproate is well recognised for its teratogenic properties and should be avoided if at all possible. However its teratogenic potential is dose dependent,7 and so keeping doses below 1000mg per day can significantly reduce this toxicity. At a daily dose of 2g daily, then risk is close to 70% but at 1g daily, this reduces to approximately 20%.7
Carbamazepine is another anticonvulsant with significant teratogenic potential but much less so than valproate but again showing a dose-dependent effect. At doses of 1g daily, carbamazepine carries a 5% risk.
As an enzyme inducer, folic acid supplementation at a dose of 5mg daily should be considered for mothers prescribed this agent.
Evidence supports lamotrigine as a safer option8 and is a treatment possibility for both bipolar depression and many types of seizure management.
Anxiolytics and tranquillisers
There is an association between benzodiazepine use in pregnancy, low birth weight and pre-term delivery.9 Other than for short term (intermittent) use (for example, rapid tranquillisation, interruption of a manic episode), regular use of benzodiazepines can lead to temperature dysregulation, lower Apgar scores, hypotonia and poor feeding in the neonate, and when used close to delivery can result in a floppy baby syndrome.10 If acute sedation with a benzodiazepine is needed close to term, then an agent with a short half-life and lower risk of accumulation (for example, oxazepam or lorazepam) is preferred.
Oxazepam is a lower potency agent compared with lorazepam and means that dose titration can be more easily fine-tuned to the patient’s needs, but the agent is only available in oral form.
The anticonvulsants gabapentin and pregabalin are sometimes used in the management of generalised anxiety disorder. Gabapentin has more information reported than pregabalin but there are insufficient data to recommend either agent under these circumstances.11,12
Poor sleep is best initially managed by use of sleep hygiene techniques but if medication is required, the sedative antihistamine promethazine is recommended in preference to the Z drugs.5
There are insufficient data to endorse absolute safety of zopiclone and zolpidem as many reports involve taking of other psychotropic agents.
Antipsychotics
As a group, antipsychotics are considered relatively safe for use during pregnancy and stopping appropriate use poses a significant risk.13 There is no evidence to suggest that second generation antipsychotics (SGAs) are different in safety profile to first generation antipsychotics (FGAs) so the choice of antipsychotic can be made on other clinical considerations.
Of the SGAs, olanzapine has been studied extensively, and studies suggest no difference in outcomes compared with the general population.14 However, consideration should be given to the metabolic profile of olanzapine, whereby an increase in insulin resistance and weight gain may more readily promote hyperglycaemia and development of gestational diabetes. The same considerations should be applied to other antipsychotics that have high metabolic burdens (for example, chlorpromazine). Olanzapine is metabolised by CYP1A2, which is downregulated during pregnancy, so the dose may need to be reduced. At term, the neonate may present with hypoglycaemia.15 Quetiapine has a relatively lower metabolic burden and risk of extrapyramidal side effects (EPS), and limited data (largely case reports) suggest no evidence of congenital anomalies or spontaneous abortion with this agent.
Low potency FGAs (for example, phenothiazines) with their intrinsic antihistaminic, anticholinergic and alpha-noradrenergic blocking properties, may contribute to postural hypotension, especially during mid-pregnancy.16
High potency FGAs (for example, haloperidol) carry less metabolic burden but an increased risk of EPS that may require co-prescription of anticholinergic agents such as procyclidine. This then introduces a second drug to the mother and foetus and the peripheral anticholinergic side effects of dry mouth, blurred vision and constipation may impact on the mother’s wellbeing. The CNS-mediated anticholinergic effects of procyclidine are likely to result in a slowed reaction time if not overtly impacting on cognitive abilities.
Depot antipsychotics are not generally encouraged as they offer little opportunity for flexibility of dose.
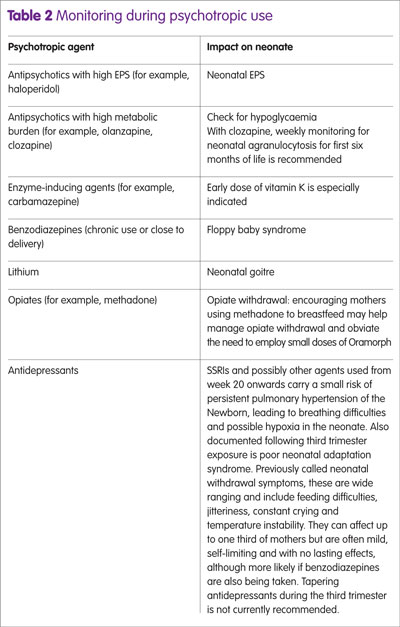
While the perinatal period is not an ideal time to initiate a patient on clozapine, patients already on clozapine are unlikely to be able to be switched to an alternative agent because it is the only therapy indicated for treatment-refractory schizophrenia. Close attention to optimisation is therefore required and this can now be helped with monitoring of clozapine blood levels. For example, if a patient stops smoking then this, in addition to physiological downregulation of CYP1A2 metabolising enzyme, will significantly affect clozapine levels. An anticipated 50% increase in blood levels can be expected on cessation of smoking because induction of this enzyme from the hydrocarbons in cigarette smoke is lost. At term, weekly monitoring of the neonate for signs of agranulocytosis is recommended for the first six months of their life.17
Many patients on antipsychotics are likely to fall into categories requiring low molecular weight heparin to reduce chances of blood clots during pregnancy. A venous thromboembolism score chart is likely to be in place to help determine the dose and duration of anticoagulation.
Use during the third trimester of any antipsychotic may lead to some transient EPS in the neonate, and withdrawal effects in 15% neonates.18
Antidepressants
The selective serotonin reuptake inhibitors (SSRIs) are the best-studied class of antidepressants and there is much evidence to support their use during pregnancy, and so SSRIs represent the first-line option for depression. In clinical practice, sertraline or fluoxetine are popular choices due to their low drug interactions and their use does not lead to additional concerns in pregnancy. Taken from week 20 onwards, all SSRIs have been linked to an increased risk of persistent pulmonary hypertension in the newborn but absolute risk is low and evidence somewhat conflicting.19
Similarly, all SSRIs have been associated with a slightly increased risk of heart defect, with possibly the highest risk with paroxetine, although this is still controversial and a meta-analysis of prospective cohort studies found no association between SSRI use in the first trimester and heart defects.20 Paroxetine is also more likely to carry increased risk of pregnancy-induced hypertension.21 Paroxetine has many drug interactions and, if doses are missed, is more prone to precipitating acute discontinuation reactions. It is therefore still probably best avoided.
Citalopram has few interactions but QTc prolongation makes it a less favourable choice, particularly in patients who have cardiac vulnerabilities. Escitalopram has a more favourable QTc profile but there is less evidence of safety during pregnancy. Both citalopram and escitalopram have a higher foetal to maternal plasma ratio than sertraline.22
However, patients already stabilised on one SSRI should not be switched to an alternative SSRI as there are reports that this leads to poorer outcomes. One study has shown that combining a benzodiazepine with an SSRI may lead to an increased risk of congenital heart defects.23
Exposure to SSRIs during the third trimester may produce mild neonatal withdrawal reactions (for example, irritability, tremor).
Patients who do not tolerate or respond to SSRIs are likely to be considered for newer agents such as the serotonin–norepinephrine reuptake inhibitors duloxetine and venlafaxine or a tricyclic agent (TCA), in line with NICE guidance. Foetal exposure to tricyclics is high and agents such as amitriptyline may prove too sedating and their anticholinergic adverse effects are unlikely to be welcome by many patients. In this respect, nortriptyline may be preferable. Use of TCAs during the third trimester is likely to lead to some neonatal withdrawal effects: agitation, irritability, seizures, respiratory distress and endocrine and metabolic disturbances. These are usually mild and self-limiting.24 Venlafaxine was initially associated with cardiac defects, anencephaly and cleft palate, and neonatal withdrawal may also occur,25,26 with second trimester exposure being associated with babies being born small for gestational age. The more recent studies do not suggest an association between venlafaxine and congenital malformations.27 There are not enough data to actively exclude any adverse pregnancy outcomes with duloxetine, but to date no increased risk of congenital malformation, preterm delivery, still birth or neonatal complications have been identified.27 Mirtazapine has not been associated with any malformations but, in common with SSRIs, has been linked to an increase in spontaneous abortion.28
Traditional monoamine oxidase inhibitors such as phenelzine and tranylcypromine are unlikely to be considered options not only because of their food and drug interactions and risk of hypertensive crisis but also the likely increased postural hypotension during pregnancy.1
Omega 3 fatty acids may be an attractive option but the evidence base is weak.29 St John’s Wort is not recommended in pregnancy and there is no robust systemic evaluation to support its use.

Lithium
Used as both a mood stabiliser and to augment antidepressants employed in unipolar depression, lithium remains a common agent within mental health.
It presents medicines management issues across all trimesters. Ebstein’s anomaly, which is a cardiac defect where the tricuspid valve is displaced towards the right ventricle, is associated with lithium use during the first trimester but incidence is now believed to be lower than early studies reported and current incidence is estimated at 1 in 1000.
This is because the early studies did not allow for other confounders.30 It remains a preferred option to avoid lithium during the first trimester where possible but during second and third trimester treatment optimisation may involve a dose increase as volume of distribution (Vd) increases. From week 36 onwards, it is recommended that measurement of lithium levels is undertaken weekly.
Lithium is routinely stopped for around 12 hours before delivery to avoid electrolyte and other physiological changes complicating treatment. Post-partum, original treatment doses should be re-instated.
Stimulants
Studies on the impact of stimulants during the perinatal period show that methylphenidate use during the first trimester is not associated with any increased risk of major congenital malformations.31 For amphetamines, data taken from a large cohort study suggest no increased risk of malformations.32
Animal studies have shown adverse foetal effects with atomoxetine at high doses but there are no human studies to establish the safety of atomoxetine in pregnancy. If atomoxetine is to be used, then the minimum effective doses should be employed. Some patients may be slow metabolisers so any clinical signs of dose-related adverse effects should prompt a review.33
Substance misuse agents
Historically methadone has been used as the opiate substitute treatment during pregnancy but there is growing evidence to support buprenorphine as a realistic alternative, both in terms of safety and also reduced duration of neonatal abstinence syndrome. At this stage, there is not enough compelling evidence to suggest switching methadone-stabilised patients over to buprenorphine.34 During pregnancy methadone pharmacokinetics are likely to change because CYP1A2 becomes downregulated, so careful checking on maintenance doses is required.
Direct data on naltrexone in pregnancy are lacking but normal birth outcomes for a small number of patients exposed to a naltrexone implant prenatally have been reported.35
Introducing naltrexone during pregnancy should be avoided because opiate withdrawal increases the risk of neonatal distress, spontaneous abortion and premature labour and delivery,35 as well as compromising any opiate-based pain relief that the mother may require during labour and post-partum.
Term and post-natal considerations
Most psychotropic drugs are continued uninterrupted during pregnancy but any increased doses should be returned to original treatment doses immediately post-partum. There is a high risk of relapse in the immediate post-partum period in some mental health conditions. For example, in women with a Bipolar 1 diagnosis, the risk of a manic episode is 23-times higher than in controls. For any new mothers developing a mental health condition requiring treatment, consideration may need to focus more on implications for breastfeeding. When it comes to ‘red flag’ drugs, carbamazepine, clozapine and lithium are the principle ones to avoid. Some authorities recommend that breastfeeding be timed to match when plasma – and, by implication, breastmilk – levels are at their lowest but this practice has been questioned as impracticable and unnecessary.36
Neonatal considerations
Alongside background morbidity in the newborn, exposure to certain psychotropic agents can be predictive of more focused neonatal monitoring and follow up. More generally, the impact of psychotropics on speech and language development remains controversial and studies recognise many confounders. Table 2 identifies some specific monitoring that may arise from particular psychotropic use.
Key points
- A mother’s wellbeing is paramount for promoting a healthy and successful birth.
- Look beyond medication to other risk factors such as poor nutrition and lifestyle to identify what supplements or interventions may be appropriate.
- Unless serious risk is identified, do not automatically stop psychotropic medication. Dose optimisation or a switch to an another agent with a lower risk in pregnancy may be alternative options.
- Remember that pharmacokinetics change during pregnancy and so some drugs (for example, lithium) may need to have the dose increased during the second and third trimesters.
- Think ahead: will current regimen present any issues if the mother wishes to breastfeed?
References
1 Chisholm MS, Payne JL. Management of psychotropic drugs during pregnancy. BMJ 2016;352:h5918.
2 Lancaster CA et al. Risk factors or depressive symptoms during pregnancy: a systematic review. Am J Obstet Gynaecol 2010;202(1):5–14.
3 Dhavliker M, Purohit P. Preconception care: dietary and lifestyle advice. Pharm J 2017;298:44–7.
4 Dempsey DA, Benowitz NL. Risks and benefits of nicotine to aid smoking cessation in pregnancy. Drug Saf 2001:24(4):277–322.
5 National Institute for Health and Care Excellence. Clinical Pathways. Antenatal and postnatal mental health. https://pathways.nice.org.uk/pathways/antenatal-and-postnatal-mental-health (accessed July 2017).
6 Pavek P, Ceckova, M, Staud F. Variation of drug kinetics in pregnancy. Curr Drug Metab 2009;10:520–9.
7 Eadie MJ. Antiepileptic drug safety in pregnancy Clinical Pharmacist 2016;8(1)24–31.
8 Campbell E et al. Malformation risks of antiepileptic drug monotherapies in pregnancy: updated results from the UK and Ireland Epilepsy and Pregnancy Registers. J Neurol Neurosurg Psychiatry 2014;85:1029–34.
9 Calderon-Margalit R et al. Risk of preterm delivery and other adverse perinatal outcomes in relation to maternal use of psychotropic medications during pregnancy. Am J Obstet Gynecol 2009;201:579.e1–8.
10 Iqbal MM, Sobhan T, Ryals T. Effects of commonly used benzodiazepines on the fetus, the neonate, and the nursing infant. Psychiatr Serv 2002;53:39–49.
11 Molgaard-Nielsen D, Hviid A. Newer-generation antiepileptic drugs and the risk of major birth defects. JAMA 2011;305:1996–2002.
12 Fujii H et al. Pregnancy outcomes following gabapentin use: results of a prospective comparative cohort study. Neurology 2013;80:1565–70.
13 Robinson GE. Treatment of schizophrenia in pregnancy and postpartum. J Popul Ther Clin Pharmacol 2012;19:e380-6.
14 Brunner E et al. Olanzapine in pregnancy and breastfeeding: a review of data from global safety surveillance. BMC Pharmacol Toxicol 2013;14:38.
15 Gentile S. Clinical utilization of atypical antipsychotics in pregnancy and lactation. Ann Pharmacother 2004;38:1265–71.
16 Seeman MV. Clinical interventions for women with schizophrenia: pregnancy. Acta Psychiatr Scand 2013;127:12–22.
17 Gentile S. Antipsychotic therapy during early and late pregnancy. A systematic review. Schizophr Bull 2010;36:518–44.
18 Habermann F et al. Atypical antipsychotic drugs and pregnancy outcome: a prospective, cohort study. J Clin Psychopharmacol 2013;33:453–62.
19 Huybrechts KF et al. Antidepressant use late in pregnancy and risk of persistent pulmonary hypertension of the newborn. JAMA 2015;313:2142–51.
20 Wang S et al. Selective serotonin reuptake inhibitors (SSRIs) and the risk of congenital heart defects: a meta-analysis of prospective cohort studies. J Am Heart Assoc 2015;4:e001681 59.
21 De Vera MA, Berard A. Antidepressant use during pregnancy and the risk of pregnancy-induced hypertension. Br J Clin Pharmacol 2012:74(2):362–9.
22 Hasser C, Brizendine L, Spielvogel A. SSRI use during pregnancy. Current Psychiatry 2006; 5(4):31–40.
23 Reis M, Kallen B. Combined use of selective serotonin reuptake inhibitors and sedatives/hypnotics during pregnancy: risk of relatively severe congenital malformations or cardiac defects. A register study. BMJ Open 2013;3:e002166.
24 Moses-Kolko E et al. Neonatal signs after late in utero exposure to serotonin reuptake inhibitors: literature review and implications for clinical applications. JAMA 2005; 293:2372–83.
25 Polen KN et al. Association between reported venlafaxine use in early pregnancy and birth defects, national birth defects prevention study, 1997-2007. Birth Defects Res A Clin Mol Teratol 2013;97(1):28–35.
26 Einarson A et al. Pregnancy outcome following gestational exposure to venlafaxine: a multicenter prospective controlled study. Am J Psychiatry 2001;158:1728–30.
27 Lassen D, Ennis ZN, Damkier P. First trimester pregnancy exposure to venlafaxine or duloxetine and risk of major congenital malformations. A systematic review. Basic Clin Pharmacol Toxicol 2016;118:32–6.
28 Djulus J et al. Exposure to mirtazapine during pregnancy: a prospective, comparative study of birth outcomes. J Clin Psychiatry 2006; 67:1280–4.
29 Freeman MP et al. An open trial of omega-3 fatty acids for depression in pregnancy. Acta Neuropsychiatr 2006;18:21–4.
30 Cohen LS et al. A reevaluation of risk of in utero exposure to lithium. JAMA 1994;271:146–50.
31 Pottegard A et al. First-trimester exposure to methylphenidate: a population-based cohort study. J Clin Psychiatry 2014;75:e88–93.
32 Golub M et al. NTP-CERHR expert panel report on the reproductive and developmental toxicity of amphetamine and methamphetamine. Birth Defects Res B Dev Reprod Toxicol 2005;74(6):471–584.
33 Briggs GG, Freeman RK, Yaffe SJ. Drugs in pregnancy and lactation, 7th edn. Philadelphia, Pa: Lippincott Williams and Wilkins;2005.
34 Minozzi S et al. Maintenance agonist treatments for opiate-dependent pregnant women. Cochrane Database Syst Rev 2013;12:CD006318.
35 Jones HE et al. Naltrexone in the treatment of opioid-dependent pregnant women: the case for a considered and measured approach to research. Addiction 2013;108:233–47.
36 Bobo WV, Yawn BP. Concise review for physicians and other clinicians: Postpartum depression. Mayo Clin Proc 2014;89:835–44.
Resources
- United Kingdom Teratology Information Service (UKTIS) incorporating TOXBASE Telephone helpline 0344 892 0909 www.uktis.org
- BUMPS (Best Use of Medicines in Pregnancy) http://www.medicinesinpregnancy.org/
- College of Mental Health Pharmacy www.cmhp.org.uk
- Choice and medication website http://www.choiceandmedication.org/cms/subscribers/?lang=en
- Psychotropic Drug Directory (2016) Bazire S. Cheltenham: Lloyd-Reinhold Communications ISBN: 9780956915634
- The Maudsley Prescribing Guidelines 12th edition (2015) David Taylor, Carol Paton, Shitij Kapur Wiley-Blackwell ISBN: 978-1-118-75460-3