Pharmacogenetics is increasingly seen as an essential tool in drug therapy and medicines optimisation. As evidence accumulates to support its use in clinical practice, pharmacists must have the requisite knowledge and skills to support and lead in implementing pharmacogenetics as part of routine healthcare. Ben McIntyre, Jessica Keen and Dr John McDermott outline the fundamental concepts and describe how pharmacogenetics is being implemented in practice.
It is widely recognised that individuals show significant variation in how they respond to medicines. Many patients will not experience a therapeutic effect at standard dosages, whereas others might be more susceptible to adverse effects or toxicity. This variation in response can be due to several factors, such as drug interactions, comorbidities, physiological status, diet or environmental factors.
Pharmacogenomics – or more specifically pharmacogenetics when discussing individual genes – is the study of how a person’s genetic profile influences their response to medicines.1 Common genetic changes in an individual’s DNA sequence may result in therapeutic failure or put them at greater risk of adverse drug reactions (ADRs).
By identifying DNA variants in genes involved in the pharmacology of a drug – often referred to as pharmacogenes2 – in many cases we can predict the chance of treatment efficacy or ADR risk, and thus optimise the patient’s treatment accordingly.
This represents a new paradigm in healthcare, moving away from the established one-size-fits-all approach to a more precise, bespoke prescribing strategy based on a person’s individual genetic profile.
How are pharmacogenes classified?
Pharmacogenes can be classified according to their protein function, which broadly falls into one of three categories2:
- Drug metabolising enzymes
- Drug transporters
- Drug targets
All of these are involved in the pharmacokinetics (PK) or pharmacodynamics (PD) of a drug.
As an example, cytochrome P450 (CYP) enzymes are responsible for the metabolism of many different drugs and variants in the genes that code for these enzymes can increase or decrease their metabolic function.
The activity of drug transporter proteins, such as OATP1B1, which plays an important role in statin uptake and elimination and is encoded by the SLCO1B1 gene, might be also affected by genetic variants. Thus, alterations in plasma concentrations of a drug or its metabolites may consequently affect therapeutic efficacy or the likelihood of developing an ADR.
Similarly, where a drug must bind to a target protein as part of its mechanism of action, a variant may affect drug–target interaction via alterations in protein structure and hence elicit a change in drug response.
The benefits of a pharmacogenetic approach
There are several hundred drug–gene pairs (a medicine which interacts with a specific gene that influences pharmacological effect) with a potentially actionable impact on prescribing and large population-level studies have shown that almost everyone will carry at least one pharmacogenetic variant.3,4 Furthermore, several studies have shown that tailoring prescribing using a pharmacogenetic-guided approach can lead to improved patient outcomes and reduced harm.5–7
More recently, a landmark randomised, controlled trial across healthcare systems in several European countries demonstrated the benefits of a pharmacogenetic panel testing strategy in reducing adverse drug reactions.8
The Pre-emptive Pharmacogenetic Testing for Preventing Adverse Drug Reactions (PREPARE) study examined the implementation of a 12-gene pharmacogenetic panel to guide prescribing of a selection of medicines with actionable drug–gene interactions, with a 30% reduction in clinically relevant ADRs observed in comparison to standard (unguided) treatment.8
The evidence suggests that the benefits of pharmacogenetics are significant, with the potential to improve care for large numbers of patients in addition to reducing medicines expenditure and wastage.
Pharmacogenetics in practice
The process of performing a pharmacogenetic test, interpreting the result and using that information to optimise a patient’s drug therapy may be unfamiliar to many pharmacy professionals.
A schematic illustration of pharmacogenetic testing for a metabolising enzyme gene (CYP2C19) is shown in Figure 1.
A sample is taken (usually either blood or saliva) and sent to a laboratory where the DNA is analysed for genetic variants, either within a single gene or across a panel of several known pharmacogenes. The raw assay result is translated and reported in a clinically relevant format; in this case, as a metaboliser phenotype, which is then used to inform a prescribing decision.
Figure 1. Summary of how pharmacogenetic testing of CYP2C19 is performed and reported in clinical practice
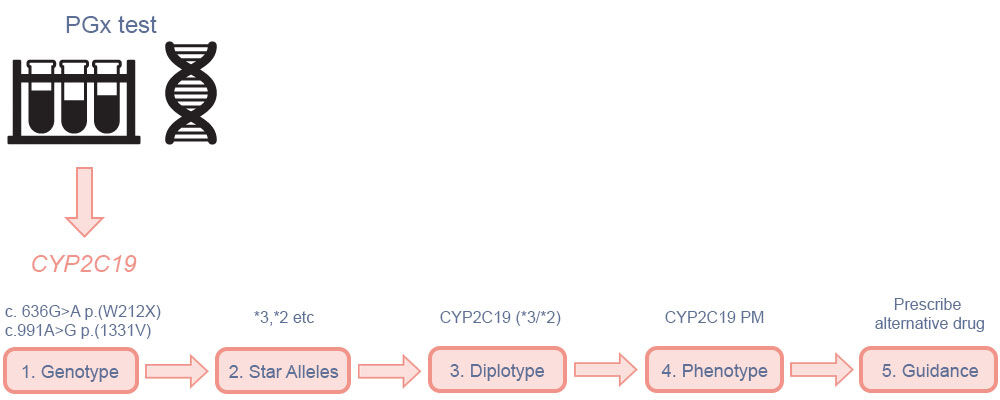
(1) The assay result reports the nucleotide variation at a certain position in the genome in comparison to a reference human genome sequence, this is known as the genotype; (2) based on the combination of variants, each copy of the gene (allele) is assigned a star allele (*1, *2 etc) according to a standardised nomenclature; (3) a diplotype is given in combination */* format, reflecting the variants inherited from each parent; (4) the combination of star alleles is translated to a metaboliser phenotype, based on whether they result in normal, increased or reduced protein function (PM = poor metaboliser); (5) pharmacogenetic dosing guidance incorporated into the prescribing decision. (PGx = pharmacogenetic/pharmacogenomic). Adapted from McDermott and Newman9
Many commonly prescribed medicines, including statins, clopidogrel, antidepressants and proton-pump inhibitors now have evidence-based guidance with actionable prescribing recommendations for clinical practice.3
Ideally, any pharmacogenetic test result would be returned to the prescriber or clinician, coupled with appropriate evidence-based prescribing advice.
The Clinical Pharmacogenetics Implementation Consortium (CPIC), which has currently published 26 peer-reviewed guidelines outlining pharmacogenetic prescribing recommendations for many medicines, and the Dutch Pharmacogenetics Working Group (DPWG) are perhaps the most prominent groups in terms of developing pharmacogenetic-based clinical guidelines.3,10
Elsewhere, the Pharmacogenomics Knowledge Base (PharmGKB) is a freely available comprehensive resource for guidelines and other pharmacogenomic-based clinical information.11
Current testing approaches
Current approaches to pharmacogenetic testing tend to be reactive, where a clinician requests a pharmacogenetic test for a patient relating to a medicine they are about to prescribe or that the patient is already taking.
An alternative, and increasingly utilised, strategy is ‘pre-emptive’ testing, where a panel of genes are genotyped in advance of any prescribing activity. As pharmacogenes are part of an individual’s inherited germline DNA, in theory, a pharmacogenetic test might only need to be performed once in a lifetime. If these results can be integrated into a patient’s medical records, they can be used to inform the prescription of future medicines throughout that patient’s life. This does however present a significant informatics challenge.
Ensuring any results are made available across a healthcare system is a challenge, particularly in systems where patients may move between different healthcare settings regularly. Any clinical pharmacogenetic service must include a robust informatics solution that ensures interoperability with all relevant electronic health records providers, so that individuals’ results and pharmacogenetic-guided prescribing information can be shared and securely accessed at any required point during patient care.
Availability of pharmacogenetic testing
The availability of pharmacogenetic tests varies across different jurisdictions and healthcare systems. Within the UK, the National Health Service Genomic Medicine Service (NHS GMS) was launched in 2018 with the aim of creating the most advanced genomic healthcare systems in the world.
Pharmacogenomics is one of the key areas of focus as part of a wider strategy to implement and accelerate the use of personalised medicine, using genetic testing to access precision treatment and optimise the use of existing medicines.12
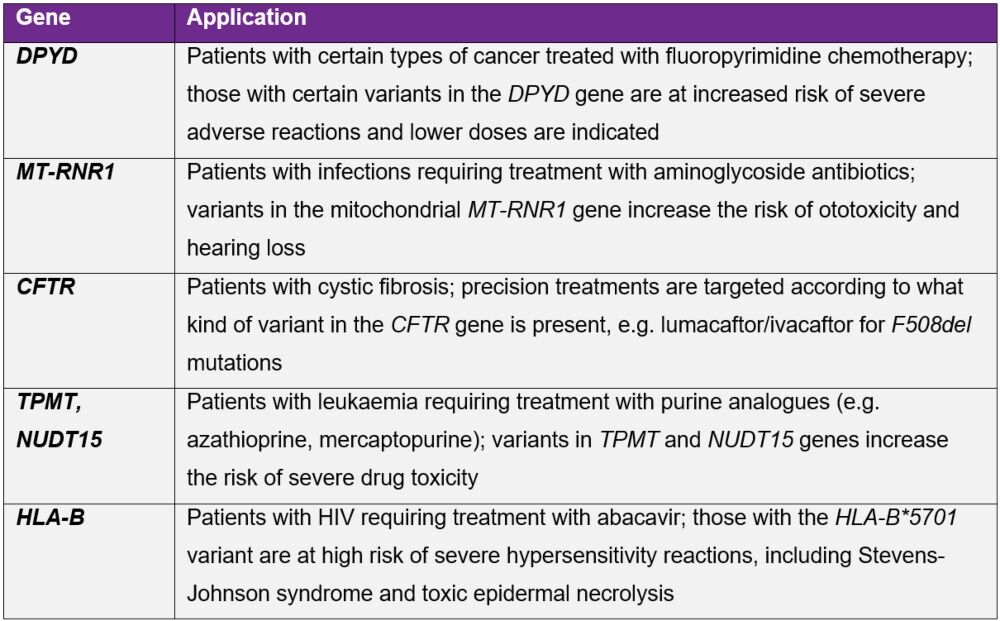
In England, the National Genomic Test Directory specifies which genomic tests are commissioned and paid for by the NHS and which patients are eligible for testing.13 Examples of pharmacogenetic testing currently performed in the UK are shown in Table 1.
Table 1. Pharmacogenetic tests currently available publicly through the NHS, showing the gene(s) tested and clinical scenarios for which these are carried out
As more clinical data and evidence become available, pharmacogenetic testing will inevitably increase and broaden in scope to include more drugs, both old and new.
For example, clopidogrel – a prodrug which is metabolised primarily by CYP2C19 to its active metabolite – is less effective in people with CYP2C19 variants, leading to reduced enzymatic activity (intermediate and poor metaboliser phenotypes). As such, individuals with these genetic variants are at increased risk of adverse cardiac and cerebrovascular events if treated with clopidogrel.7,14,15
Although this is noted as a caution or warning by UK, European and US medicines regulators, testing is not routinely undertaken in many healthcare systems.16–18
Pharmacogenetic testing can identify high-risk patients who may then be offered alternative antiplatelet treatment. Because certain variants will vary in prevalence between different ancestral populations, some patient cohorts may be at greater risk of adverse events and the benefits of pharmacogenetic testing may be even more significant.14
Genotyping of patients before initiating clopidogrel following a stroke or transient ischaemic attack is currently being evaluated for implementation into the NHS.18
CYP2C19 genotyping is currently available to guide prescribing of another medicine in the UK. Mavacamten (brand name Camzyos) is a novel cardiac myosin inhibitor recently approved for the treatment of obstructive hypertrophic cardiomyopathy. The EMA and MHRA have determined that CYP2C19 genotyping is necessary before starting treatment.
Clinical trial data suggests poor metaboliser phenotypes have significantly higher mavacamten exposure, increasing the risk of systolic dysfunction, and therefore require a lower starting and maintenance dose.20
Healthcare providers will need to have pharmacogenetic testing pathways in place to ensure not only the safe and effective use of this medicine, but also strategies to potentially re-use this CYP2C19 result to guide future prescribing.
The role of the pharmacist in pharmacogenetics
As professional experts in medicines, pharmacists will have an essential role in implementing and delivering pharmacogenetics in routine clinical care. Their training and skills enable them to interpret and communicate complex medicines-related information to patients and other health professionals.
This should be viewed as a natural extension to what they already do, with pharmacogenetic information used as another tool for medicines optimisation, rather than a dramatic shift in practice. In several countries where pharmacogenetics is already in use, pharmacists routinely use them as part of their clinical practice and often lead services.21,22
The potential roles are varied but range from daily clinical activity such as ordering tests, interpreting results and counselling patients, to medicines optimisation, education, leadership and pharmacogenetics-based research.
Some of the current and potential future roles for pharmacists involving pharmacogenetics (PGx) and genomic medicine include23,24:
- Identification of eligible or suitable patients for PGx testing as part of medicines reconciliation or medicines use reviews
- Discussing PGx test results with patients and providing appropriate counselling
- Providing expert PGx-guided medicines advice as part of the wider MDT
- Developing PGx-based clinical workflows and guidelines
- Leading on teaching, education and training for pharmacy and other healthcare professionals
- Using PGx to inform medicines governance, safety and optimisation within healthcare organisations
- Supporting or leading on PGx-based research, service evaluation and improvement initiatives
- Advising and informing pharmacy professional and educational standards.
Nevertheless, there remains an education and knowledge gap within the profession around genomic medicine generally, which could be a significant barrier to any service implementation.
Recent surveys of pharmacy professionals (both pharmacists and pharmacy technicians) in the UK have shown that, while the vast majority expect pharmacogenomics to influence or become part of their future role, confidence is low and only 10% felt adequately prepared for the anticipated changes to their practice.21
Similarly, the majority of those surveyed had never received any specific training in pharmacogenomics or genomic medicine.21,25 It is therefore essential to ensure the pharmacy workforce is sufficiently prepared to integrate pharmacogenetics into their clinical practice.
In England, educational bodies such as the Centre for Pharmacy Postgraduate Education and NHS England Genomics Education have recognised this with a variety of learning and development resources now available to pharmacists and other healthcare professionals.
These range from short e-learning and online materials to more comprehensive modules and formally taught courses up to Master’s level.26,27
Conclusion
Pharmacogenetics is increasingly recognised as a significant factor in how individuals respond differently to medicines and can serve as an essential tool to improve the safety and efficacy of drug treatment. As pharmacogenetic testing is more widely implemented in healthcare systems and becomes embedded into routine care, it will become ever more relevant to almost all areas of pharmacy practice.
Although many pharmacists do not necessarily need to be experts in pharmacogenetics, they should have an appropriate level of skill and understanding that is relevant to their role.
Pharmacists have already established themselves as experts in medicines and are thus uniquely placed to ensure pharmacogenetics is implemented effectively across healthcare settings at all levels.
This will be crucial to maximising the benefits of this new era of precision medicine, ultimately leading to better patient care and improved health outcomes.
Authors
Ben McIntyre MSc MRPharmS
NHS North West Genomic Medicine Service Alliance, UK
Jessica Keen MSc MRPharmS
NHS North West Genomic Medicine Service Alliance, UK; Manchester Centre for Genomic Medicine, St Mary’s Hospital, Manchester University NHS Foundation Trust; School of Health Sciences, The University of Manchester, Manchester, UK
John H McDermott MRCP (London) MBChB BSc
Manchester Centre for Genomic Medicine, St Mary’s Hospital, Manchester University NHS Foundation Trust, Manchester, UK; Division of Evolution, Infection and Genomics, School of Biological Sciences, The University of Manchester, Manchester, UK
References
- Turner RM, Magavern EF, Pirmohamed M. Pharmacogenomics: Relevance and opportunities for clinical pharmacology. Br J Clin Pharmacol 2022 Sep;88(9):3943–6
- Katara P, Yadav A. Pharmacogenes (PGx-genes): Current understanding and future directions. Gene 2019 Nov 15;718:144050
- Clinical Pharmacogenetics Implementation Consortium. Guidelines [online]
- McInnes G et al. Pharmacogenetics at Scale: An Analysis of the UK Biobank. Clin Pharmacol Ther 2021 Jun;109(6):1528–37
- Pirmohamed M et al. A randomized trial of genotype-guided dosing of warfarin. N Engl J Med 2013;369(24):2294–303
- Henricks LM et al. DPYD genotype-guided dose individualisation of fluoropyrimidine therapy in patients with cancer: a prospective safety analysis. Lancet Oncol 2018;19:1459–67
- Claassens DMF et al. A genotype-guided strategy for oral P2Y(12) inhibitors in primary PCI. N Engl J Med2019;381:1621–31
- Swen JJ et al. A 12-gene pharmacogenetic panel to prevent adverse drug reactions: An open-label, multicentre, controlled, cluster-randomised crossover implementation study. Lancet 2023;401(10374):347–56
- McDermott JH, Newman W. Introduction to pharmacogenetics. Drug Ther Bull 2023 Nov;61(11):168–72
- Koninklijke Nederlandse Maatschappij ter bevordering der Pharmacie. Farmacogentica [online]
- PharmGKB [online]. PharmGKB. 2024
- NHS England. Accelerating genomic medicine in the NHS [online]
- NHS England. National genomic test directory [online]
- Magavern EF et al. CYP2C19 Genotype Prevalence and Association With Recurrent Myocardial Infarction in British-South Asians Treated With Clopidogrel. JACC Adv 2023 Sep;2(7):100573.
- Wang Y et al. Association between CYP2C19 loss-of-function allele status and efficacy of clopidogrel for risk reduction among patients with minor stroke or transient ischemic attack. JAMA 2016;316(1):70
- Sanofi. Plavix 75mg tablets – Summary of Product Characteristics [online]. Electronic Medicines Compendium, 2023
- Sanofi-Aventis. Plavix – Summary of Product Characteristics [online]. European Medicines Agency, 2023
- Sanofi-Aventis. Plavix tablets – Highlights of prescribing information [online]. Food and Drug Administration, 2022
- National Institute for Health and Care Excellence. Clopidogrel genotype testing after ischaemic stroke or transient ischaemic attack. [online]
- Bristol Mayers Squibb. Camzyos 10 mg hard capsules – Summary of Product Characteristics [online]. Electronic Medicines Compendium, 2023
- Burns C. Are pharmacists ready for the genomic revolution? Pharm J2022;7960:308(7960)
- McDermott JH et al. Characterizing pharmacogenetic programs using the consolidated framework for implementation research: A structured scoping review. Front Med (Lausanne) 2022;18;9:945352
- Royal Pharmaceutical Society. The Role of Pharmacy in Pharmacogenomics [online]. RPS, 2023
- Royal Pharmaceutical Society. Collaborative Position statement for Pharmacy professionals and Genomic Medicine [online]
- Wickens HJ et al. Pharmacy and genomic medicine: A UK-wide survey of Pharmacy staff assessing their prior education, confidence and educational needs. Int J Pharm Pract 2023;31(Supplement_2):ii53–ii53
- Centre for Pharmacy Postgraduate Education. Introduction to genomics in pharmacy. [online]
- NHS England. Genomics Education Programme. [online].