teaser
British researchers have discovered a protein mechanism which regulates the activity of cells that destroy bone tissue and a constituent in cannabis that can stop the breakdown.
The study by a team of scientists from the University of Aberdeen could potentially lead to newer treatments for bone diseases such as osteoporosis.
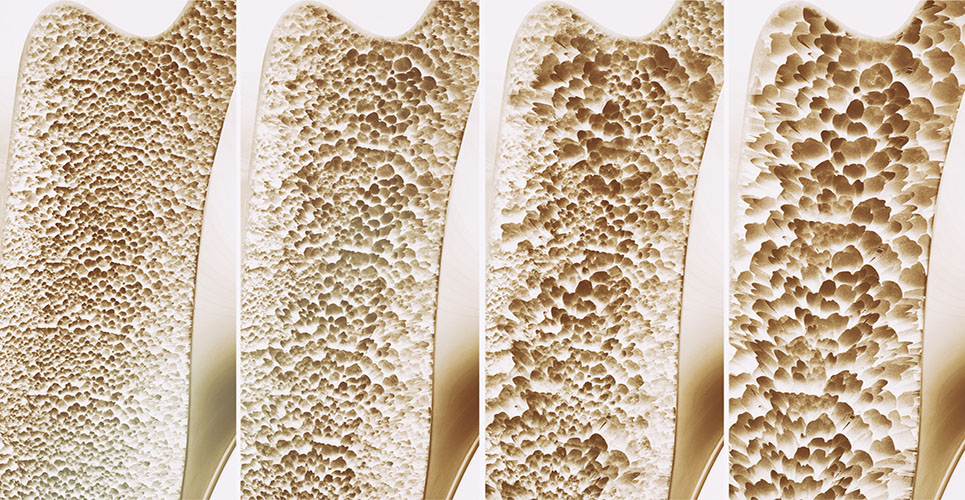
Normally bone tissue repairs itself through the activities of bone-forming osteoblasts and bone-reabsorbing osteoclasts. As people grow old osteoclasts begin to outnumber osteoblasts and bone density decreases.
A protein known as GPR55 found in bone cells has now been shown to regulate osteoclasts. Studies showed that activation of GPR55 caused the osteoclasts to destroy more bone, while blocking the protein stopped the cells from breaking down tissue.
The researchers also found that cannabidiol or CBD – a compound found in cannabis but not linked to the ‘high’ associated with smoking it – can block GPR55. They used a synthetic version of CBD for the lab tests.
One of the study leaders, Professor Mike Rogers, said: “We have shown for the first time a link between this particular protein and the activity of cells which eat away at our bones.
We are also first to show that blocking GPR55 may have direct beneficial effects on bone cells in certain bone diseases.”
But the researchers have insisted that smoking cannabis will not help fight conditions such as osteoporosis.

The findings are published in the Proceedings of the National Academy of Sciences.
Copyright © Press Association 2009
<http://www.pnas.org/> (Proceedings of the National Academy of Sciences)
NICE rejects liver cancer treatment
A liver cancer drug has been rejected for use on the NHS by the UK’s cost-effectiveness healthcare watchdog, even though the drug company has lowered the cost of treatment.
Nexavar, the drug generically known as sorafenib, was designed to treat hepatocellular carcinoma, the most common form of liver cancer which affects between 80 and 90% of primary tumours.
The National Institute of Health and Clinical Excellence (NICE) previously refused to accept the drug, created by Bayer, for treating kidney cancers. This is the second time it has turned down Nexavar for liver treatment, as it initially refused to approve it in May, but Bayer tried to reduce the cost of the drug to the NHS by putting forward an patient access scheme.
NICE was unconvinced, which is a huge setback to Bayer and its partner Onyx Pharmaceuticals, who have high hopes for Nexavar and anti-blood clotting pill Xarelto. Bayer has proven the Nexavar works for liver and kidney cancer, and are seeking to use it on breast and lung tumours.