Infants born prematurely are at considerable nutritional risk. Attention is increasingly focused on optimising the nutritional management of preterm infants to improve long-term health and outcomes.
The goal of the nutritional management of the preterm infant is to achieve a postnatal growth rate and body composition approximating that of the foetus of the same gestational age,1 and obtain a functional outcome comparable to infants born at term.2 Given that most foetal nutrient stores are deposited during the last three months of pregnancy, preterm infants have minimal energy and nutrient reserves. In a preterm infant weighing 1kg, fat contributes only 1% of total body weight,3 as compared with a term infant weighing approximately 3.5kg with 11% body fat.4 Without nutrition, a small preterm baby has sufficient reserve to survive only four days of starvation, and a large preterm baby has enough for 12 days.5
An overview of parenteral nutrition in preterm infants
Preterm delivery is considered a nutritional emergency, and provision of parenteral nutrition (PN) has become an integral part of the clinical management of preterm infants.6 The preterm infant is initially dependent on receiving nutrients parenterally due to the immaturity of the gastrointestinal tract precluding the digestion and absorption of adequate nutrients.
The majority of preterm infants born less than 30 weeks’ gestation require PN for a period of time, the duration of which is determined by gestation, birth weight and presence of co-morbidities.3 This period is followed by the subsequent initiation and advancement of enteral nutrition (EN), with PN used to support the transition to full EN, and has recently been reported to last from seven to ten days.7,8 PN should be continued until adequate nutrient intakes are achieved from EN, that is, until a minimum of 75% of the nutrient requirement is tolerated enterally.9
PN can also be provided as a sole source of nutrition when EN is contraindicated, for example, for infants with necrotising enterocolitis. While there have been considerable advances in the nutritional management of the preterm infant, nutrient deficits,7,10,11 and growth failure12–14 continue to be reported.
Types of PN
Preterm PN can be provided as a standard, readily available commercial formulation termed as standardised PN (SPN). This type of PN can also be referred to as ‘stock PN’ or a ‘stock bag’. SPN contains a fixed amount of nutrients that cannot be altered. SPN can be provided as two solutions, a ‘2-in-1’ aqueous solution containing amino acids and glucose, with or without electrolytes, trace elements and vitamins, and a separate lipid solution.
Alternatively, SPN is available as an ‘all in one’ solution, that is, a ‘3-in-1’ macronutrient solution containing amino acids, glucose and lipid, and this solution may also contain electrolytes, trace elements and vitamins. PN can also be provided as individualised PN (IPN), which is also known as patient-specific PN, that is individually prescribed nutrients and electrolytes specific to each infant’s requirements.
This type of PN involves a daily prescription for each individual preterm infant. The IPN bag is then manufactured based on this daily prescription from its basic components, for example, amino acids, glucose, lipid, electrolytes, vitamin and trace element solutions.
Traditionally, IPN has been considered the ‘gold standard’ in preterm PN for achieving optimal nutrient intakes and for fluid and electrolyte balance.15,16 However, evidence also suggests that preterm infants can be managed on SPN with better nutrient provision,17-19 rapid availability,18,20 fewer prescription and administration errors,21 possible reduction in infection risk,22 and cost savings18,23,24 being cited.
Recent advances in SPN include firstly concentrated formulations (nutrients provided in smaller volumes) which help deliver optimal nutrition despite fluid restriction or concomitant medication use, and secondly, some more nutritionally complete formulations are suitable for longer-term use. Finally, the use of standardised macronutrient regimens with daily individualised electrolyte additions can offer the flexibility of IPN with potentially reduced risks related to compounding.25,26
These improvements are driving an increasing use of SPN in preterm infants.18,25,27,28 It has been proposed that SPN can manage 80% of preterm PN prescriptions;29 however, there will continue to be a role for IPN in the more nutritionally complex and fluid restricted preterm infants.
Indications for IPN
This section provides an overview of the specific indications for IPN.
Fluid restriction and electrolyte management
Preterm PN has often to balance two competing priorities: (i) optimal nutrient provision; and (ii), flexibility for fluid and electrolyte management, which can be complex and have a rapidly changing course. The fluid management in preterm infants can be very complex, aiming to optimise nutrition while achieving homeostasis, against a background of high insensible water losses through immature skin, immature renal function affected by concomitant respiratory disease, and avoidance of fluid overload exacerbating a haemodynamic effect on patent ductus arteriosus and evolution of neonatal chronic lung disease. IPN enables the consideration of these factors when calculating fluid requirements for PN, thus allowing the delivery of nutrients in a smaller volume, that is, a more concentrated volume than is available from SPN formulations, enabling improved nutrition in extremely fluid restricted infants. Some preterm infants can have widely varying electrolyte requirements that require IPN for optimal management.
Nutrient intolerance
Another important indication for IPN is nutrient intolerance. Infants have very different abilities to manage nutrients such as glucose and lipid. Hyperglycaemia is a common complication of preterm PN, requiring a reduction in glucose delivery and/or treatment with insulin. The tolerance and clearance of lipid infusions increases with increasing gestation and can be impacted by high lipid doses, sepsis, if critically ill, if extremely low birth weight, if severe unexplained thrombocytopenia3 or if significant acidosis. Reducing SPN volumes to manage nutrient intolerance can impact on the provision of other nutrients, for example, amino acids which can be an indication for IPN.
Long-term PN management
Preterm infants receiving PN for longer than three weeks, for example, infants post-bowel resection, should be considered for additional supplementation of iron and zinc, which may not be routinely added to SPN regimens. IPN offers the flexibility to meet the longer term nutritional needs of the preterm infant.
Requirement for acetate
The preterm infant is subject to numerous conditions that can disturb acid–base homeostasis. Mild persistent metabolic acidosis can be managed by replacing choride in IPN with acetate, that is acetate salts can replace chloride salts of sodium/potassium. It has been reported that 6–7mmol/kg/d can be added as needed to maintain acid–base balance.30 Many SPN bags do not contain acetate and some preterm infants may require daily individualised acetate prescriptions.
Provision of IPN
An overview on the process of providing IPN to a preterm infant in the neonatal unit setting, including prescribing and manufacturing, is given.
Who prescribes IPN?
The prescribing of IPN is a multidisciplinary team process ideally with a co-ordinated team of clinicians, dietitians, pharmacists, and nurses preferably operating within a nutrition team, and working with appropriately trained and experienced clinical ward staff.31–33
How is IPN prescribed?
IPN is prescribed daily and should include the assessment of the infant’s medications, clinical status, biochemistry results, fluid balance, enteral feed tolerance and nutrient requirements to ensure an optimal IPN order.32 Depending on the hospital, IPN is ordered using a paper based form or an electronic PN calculator. Figure 1 displays an example of computerised PN prescribing.

PN fluid volume prescribing
Determining the volume of fluid (ml/kg/d) available for PN should be the first decision point in the prescribing process to ensure optimal nutrient delivery. IPN is commonly prescribed as if all nutrition is via the parenteral route (that is, not taking enteral feeds into account) and based on the predicted fluid allowance at the time of prescribing. This ensures there will be sufficient PN if enteral feeds are not tolerated and need to be reduced or stopped.
The volume used for IPN prescribing is equal to the volume available for nutrition (PN + EN), that is, the total fluid volume minus volume of non-nutritional parenteral fluids (IV infusions or drugs). Alternatively, IPN can be ordered in the anticipated volume available for PN only (excluding both non-nutritional parenteral fluids and enteral feeds). Once the PN volume is determined, it is generally prescribed as two phases: (i) lipid; and (ii), aqueous.
The lipid phase is a fixed concentration (for example, 20% lipid solution) so the volume is determined by the amount of lipid ordered, for example, 1g = 5ml, and can be ordered with or without vitamins. The remaining PN volume is available for the aqueous phase which contains amino acids, glucose, electrolytes with or without trace elements and vitamins.
Nutrient and electrolyte prescribing
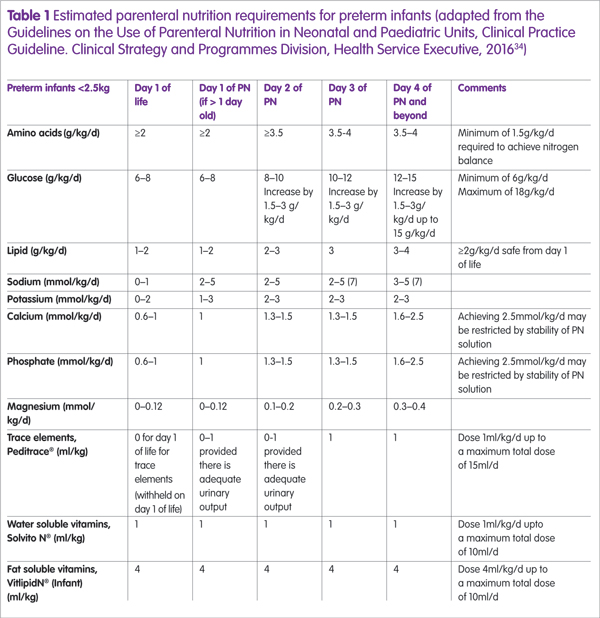
Nutrients such as amino acids, glucose and lipid are advanced gradually and the estimated PN requirements for preterm infants are described in Table 1, which can be used by neonatal units to guide nutrient advancement.34 It is important to ensure that the glucose concentration (g/100ml aqueous phase) is safe according to line access availability, with the maximum concentration for peripheral access being 12.5% (12.5g/100ml);3 concentrations higher than this must be given centrally. Consideration should also be given to the other constituents of the IPN regimen (that is, amino acids and electrolytes) because they contribute to the overall osmolarity of the solution and PN osmolarity >900mOsm/l requires infusion via a central line.30
How is IPN manufactured?
Once the IPN prescription has been written, it is transmitted for compounding. IPN manufacturing practices vary, with some countries such as UK and France having a legal requirement to manufacture PN in a pharmacy.35 In others, such as Germany and Belgium, PN can manufactured either in the ward or in the pharmacy. In the Republic of Ireland, hospital pharmacies do not have capacity to manufacture preterm PN and all manufacture is outsourced to a single, centralised commercial manufacturer. Ideally, IPN solutions should be prepared every day with strict aseptic techniques, in the pharmacy or local PN compounding unit, and not at ward level.
The future
IPN is essential for the provision of appropriate nutrient delivery, and management of fluid and electrolyte balance in a cohort of preterm infants. However, an important limitation is the daily requirement for a complete bespoke prescription which can be resource intensive and can be prone to medical errors with a lack of experienced prescribers.25
Computerised prescribing of IPN has been shown to overcome some of these shortcomings and should be considered as an important advance in IPN prescribing.25 In addition, we agree with recent recommendations32 that this process should be supported by multidisciplinary nutrition teams to ensure optimal IPN delivery for this vulnerable patient group.
Key points
- Preterm delivery is considered a nutritional emergency requiring the provision of parenteral nutrition (PN)
- The use of standarised parenteral nutrition is increasing but there is an ongoing role for individualised parenteral nutrition (IPN) in a cohort of preterm infants
- Indications for IPN include fluid restriction, for example, extremely preterm infant and electrolyte management, nutrient intolerance, long term PN and acetate requirement
- IPN is prescribed daily and should include the assessment of the infant’s medications, clinical status, biochemistry results, fluid balance, enteral feed tolerance and nutrient requirements to ensure an optimal IPN order
- IPN should be prescribed by a multidisciplinary team ideally with a co-ordinated team of clinicians, dietitians, pharmacists, and nurses preferably operating within a nutrition team, and working with appropriately trained and experienced clinical ward staff.
