teaser
More than 2,000 children in the UK who suffer from a painful form of arthritis could be treated with a drug currently used only by adults within the next two years, a pharmaceutical company has said.
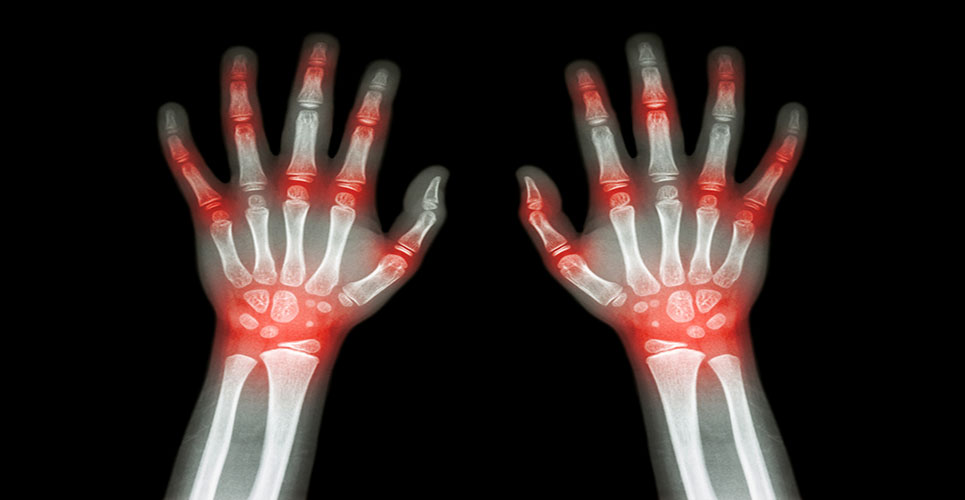
Systemic juvenile idiopathic arthritis (JIA), also known as Still’s disease, affects about 2,500 British children. In a recent study, 90% of JIA sufferers who took antibody drug tocilizumab, currently licensed for adults with moderate to severe rheumatoid arthritis, improved by up to 90% after three months.
Manufacturer Roche hopes that tocilizumab, marketed as RoActemra, will be approved for children in the UK with systemic JIA by 2012. Health experts think systemic JIA is an auto-immune condition, like adult rheumatoid arthritis, and they will monitor children involved in the research for five years to assess any long-term effects of treatment.
The study outcomes were presented at the European League Against Rheumatism (Eular) annual meeting in Rome.
Rheumatology expert Professor Patricia Woo, from Great Ormond Street children’s hospital in London, who led one of the UK arms of the trial, said: “This is a major advance for these young people.”
Systemic JIA sufferers have high levels of an immune system cell-signalling molecule called IL-6 in their blood and joints. Tocilizumab works by blocking the IL-6 biological pathway, preventing its signals from getting through.
Copyright Press Association 2010
European League Against Rheumatism