Psoriasis is a chronic inflammatory skin condition, characterised by inflamed, thickened, silver-white plaques, that is thought to affect up to 3% of the UK population.1 Although not life-threatening, psoriasis is associated with a significant impairment of quality of life2 and emotional well-being.3 Although there are several different presentations of psoriasis,2 the most common, affecting around 90% of patients, is the chronic plaque form.4
Psoriasis is a chronic inflammatory skin condition, characterised by inflamed, thickened, silver-white plaques, that is thought to affect up to 3% of the UK population.1 Although not life-threatening, psoriasis is associated with a significant impairment of quality of life2 and emotional well-being.3 Although there are several different presentations of psoriasis,2 the most common, affecting around 90% of patients, is the chronic plaque form.4
A consideration of disease severity helps guide practitioners to select the most appropriate treatment modality. However, there are currently several different tools available including the psoriasis and area severity index (PASI), body surface area (BSA) and the dermatology quality of life index (DLQI), which provides insight into the impact of the disease. All three tools have advantages yet, until recently, there was a lack of agreement on which one to use. In 2011, a consensus group dichotomised disease severity into either mild or moderate to severe using all three measures. For example, psoriasis was deemed mild when all three measures, that is, BSA, PASI and DLQI, were < 10 and moderate to severe when each tool was >10.5
Biologic treatments
In the past ten years, biologics have revolutionised the management of patients with severe psoriasis unresponsive to conventional treatments.6 Although patients with mild disease are usually managed with topical agents and those with more severe disease given systemic treatments, a biologic is considered when either approach fails to achieve a satisfactory clearance with approximately 20% of patients having moderate to severe disease.7
In 2017, the British Association of Dermatologists (BAD) updated their guidelines for the use of biologic agents8 and recommend that a biologic agent should be used where:
- Disease severity is severe, that is, PASI > 10, BSA and a DLQI > 10
- If phototherapy and alternative standard therapies are contraindicated or cannot be used
- Where patients have failed to respond to systemic therapies.
Currently, several agents are available which target different pathological factors in the aetiology of psoriasis. For example, infliximab, etanercept and adalimumab, inhibit tumour necrosis factor alpha, whereas ustekinumab, blocks the action of the pro-inflammatory cytokines interleukin (IL)-12 and -23.
Recently three monoclonal antibodies (mAbs) that target a different pathway on psoriasis have been introduced: brodalumab; secukinumab and izekizumab.
The IL-17 pathway
Psoriatic plaques contain a high level of T helper cells, in particular Th1 and Th2, although another class of T helper cells have been discovered. Known as Th17, these cells produce two ILs – IL-17A and IL-17F – that play an important role in the immune response to bacteria and fungi. In fact, there are known to be at least six distinct but structurally-related IL17 cytokines (named ILA to ILF) but their precise function remains to be determined.
In addition to an important immune function, it has also been established that overexpression of IL-17A and IL-17F contributes to the development of several autoimmune inflammatory diseases. Indeed, elevated levels of IL-17A have been found in the serum and lesional skin of those with psoriasis and in the synovial fluid of individuals with rheumatoid arthritis. It appears that both IL-17A and IL-17F and to some extent the other interleukins (for example, IL-17C, IL-17E) exert their effect through binding with a IL-17 receptor (IL-17RA) that is present on many cell types including macrophages, neutrophils, fibroblasts, epithelial cells and keratinocytes. Activation of the receptor stimulates the production and release of even more pro-inflammatory cytokines and thus drives hyper proliferation of keratinocytes.
Both secukinumab and izekizumab inhibit the action of IL-17 whereas brodalumab is somewhat unique in that it blocks the IL-17RA, thus affecting the actions of other IL-17 cytokines.
Clinical studies
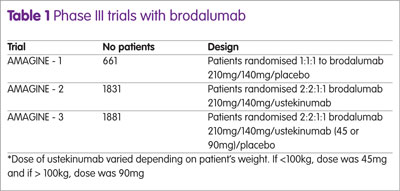
The efficacy of brodalumab in the treatment of patients with moderate to severe psoriasis has been studied in three Phase III trials: AMAGINE-1, -2 and -3. AMAGINE-1 was a placebo trial, whereas AMAGINE-2 and 3 used ustekinumab as a comparator.
A summary of the trial designs are shown in Table 1. The drugs were administered subcutaneously on day 1 and thereafter on weeks 1, 2, 4, 6, 8 and 10. AMAGINE -1 used a co-primary end-point of PASI 75 and static Physician Global Assessment (sPGA) of 0 or 1 at week 12 which represents clear (0) or almost clear (1).
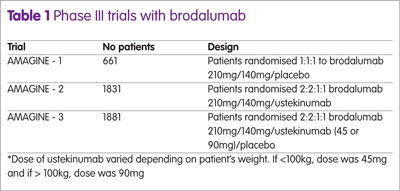
Secondary end-points were PASI 90, PASI 100 and the Psoriasis Symptom Inventory (PSI). The PSI is a validated self-rated symptom assessment tool that evaluates eight features of psoriasis; itch, redness, scaling, burning, cracking, stinging, flaking and pain. Each item is scored on a 5-point scale from 0, representing not at all to 4, defined as very severe. The maximum score is 32 and for the purposes of the trials, a PSI ‘response’ was defined as a total score not greater than 8 with no item on the scale scoring more than 1.
At 12 weeks, patients receiving brodalumab were re-randomised to receive either placebo, brodalumab 210mg or brodalumab 140mg every two weeks through to week 52. In AMAGINE – 2, at week 12, patients given brodalumab were re-randomised in a 2:2:2:1 fashion to receive brodalumab 210mg every two weeks, 140mg every two weeks, 140mg every four weeks or 140mg every eight weeks through to week 52. Patients originally given placebo were changed to brodalumab 210 mg every two weeks to week 52. Those given ustekinumab remained on the same dose throughout. This same protocol was adopted for AMAGINE-3. A further secondary end-point in AMAGINE-2 was PASI 100 in patients taking brodalumab versus ustekinumab.
The proportion of patients achieving a PASI 75, 90 and 100 for the different doses of brodalumab and ustekinumab at 12 weeks are shown in Figure 1 and the number achieving a PSI response and a sPGA of 0 or 1 in Figure 2.
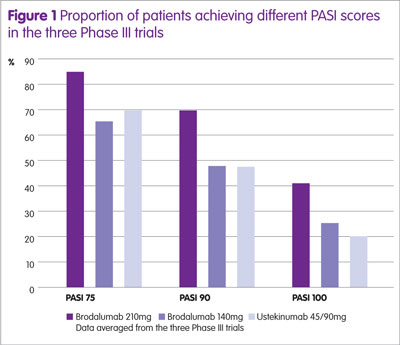
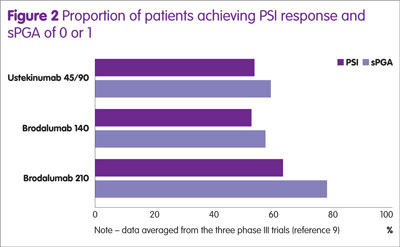
The proportion of patients achieving a PASI 75, PASI 90 and PASI 100 was significantly higher for brodalumab compared with ustekinumab (p < 0.01). Furthermore, the proportion of patients achieving a sPGA response was also greater for brodalumab compared with ustekinumab (p < 0.05).
Adverse effects
Pooled data from the three AMAGINE trials suggest that 57.6% of patients experienced an adverse event at a dose of 210 mg, 55.6% at the 140mg dose compared to 51% taking placebo.9 The most frequently reported events included nasopharyngitis, upper respiratory tract infections and arthralgia.
Long-term efficacy
The AMAGINE-2 and -3 trials provided 52-week efficacy data that demonstrated maintained efficacy for sPGA only. However, a recent, 52-week open-label trial in 133 Japanese patients found that the study end, the proportion of patients with a PASI 75, PASI 90 and PASI 100 was 94.4%, 87.5% and 55.6% respectively.10
Place in therapy and conclusions
The latest BAD guidelines recommend that patients are initiated on ustekinumab as a first-line agent or adalimumab if the person has psoriatic arthropathy. This is likely to reflect the fact that Ustekinumab has been available for many years and is aligned with the appraisal conducted by the National Institute for Health and Care Excellence (NICE) in 200911 and for adalimumab in 2007 for rheumatoid arthritis. However, secukinumab can also be used as a first-line therapy in people with or without psoriatic arthritis, based on an assessment by NICE in 2015.12
While this latest guideline does appear to recognise that the newer IL-17 inhibitors have a role, the evidence recommendation is defined as ‘weak’ and this is likely to reflect the lack of experimental data on the drug. Nevertheless, brodalumab has been granted a marketing authorisation by the European Medicines Agency13 and is scheduled to be reviewed by NICE. Until a full Summary of Product Characteristics is available and clinicians are able to use the product, the position of brodalumab remains to be determined. Nonetheless, the promising data from Phase III studies and its superiority to ustekinumab means that in the future, brodalumab might become a key treatment for the management of those with moderate to severe psoriasis.
Key points
- Psoriasis is a chronic skin condition affecting up to 3% of the UK population.
- Around 20% of patients with psoriasis have moderate to severe disease that warrants a biologic treatment when oral therapy fails.
- Brodalumab is a monoclonal antibody recently granted approval by the European Medicines Agency.
- Brodalumab targets a different pathologic pathway to currently available biologics.
- It has been shown to be superior to ustekinumab in two Phase III trials.
References
1 Psoriasis Association. About psoriasis. www.psoriasis-association.org.uk/pages/view/about-psoriasis (accessed July 2017).
2 Langley RGB et al. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis 2005;64 (Suppl 2):18–23.
3 Kimball AB et al. The psychosocial burden of psoriasis. Am J Clin Dermatol 2005;6(6):383–92.
4 Griffiths CEM et al. A classification of psoriasis vulgaris according to phenotype. Br J Dermatol 2007; 156:258–62.
5 Mrowietz U et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res 2011;303:1–10.
6 Tucker R. Biologics in moderate to severe plaque psoriasis. Hospital Pharmacy Europe 2014;72:53–5.
7 Menter A et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. J Am Acad Dermatol 2011;65 137–74.
8 Smith CH et al. British Association of Dermatologists’ guidelines for biologic interventions for psoriasis 2017. Br J Dermatol 2017; May 17 [Epub ahead of print].
9 Farahnik B et al. Brodalumab for the treatment of psoriasis: a review of phase III trials. Dermatol Ther (Heidelb) 2016;6:111–24.
10 Umezawa Y et al. Long-term clinical safety and efficacy of brodalumab in the treatment of Japanese patients with moderate-to-severe plaque psoriasis. JEADV 2016;30:1957–60.
11 National Institute for Health and Care Excellence. Ustekinumab for the treatment of adults with moderate to severe psoriasis. TA180. www.nice.org.uk/guidance/ta180/chapter/1-Guidance (accessed July 2017).
12 National Institute for Health and Care Excellence.Secukinumab for treating moderate to severe plaque psoriasis. TA 350. www.nice.org.uk/guidance/ta350 (accessed July 2017).
13 European Medicines Agency. Kyntheum. www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/003959/smops/Positive/human_smop_001146.jsp&mid=WC0 b01ac058001d127 (accessed July 2017).