teaser
Lyn C Guenther
MD FRCPC
Professor of Dermatology
University of Western Ontario
London, Ontario
Canada
E:[email protected]
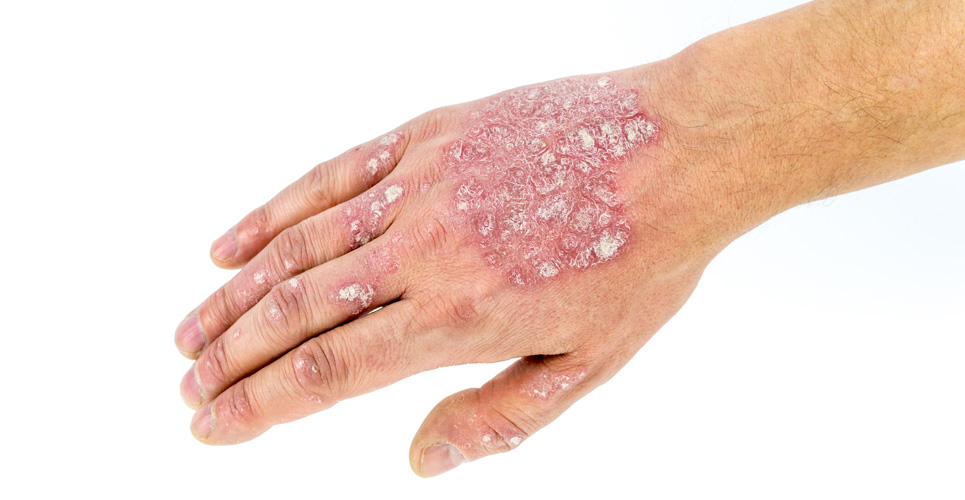
Psoriasis is a common, chronic, recalcitrant, emotionally and physically disabling condition with a prevalence rate of approximately 2%.(1) It is a T-cell-mediated inflammatory disease characterised by epidermal hyperproliferation, abnormal keratinisation and local vascular changes.(2) Treatment should be individualised. Topical therapy is first-line treatment for localised disease. Phototherapy, traditional systemic therapy and biological agents are indicated for disease affecting >10% of the body surface area (BSA), disease resistant to topical therapy and psoriasis having a significant impact on quality of life (eg, functional impairment, marked discomfort or pruritus and psoriasis in visually prominent locations).(3) In a 1998 patient membership survey from the National Psoriasis Foundation, one-third of the patients with severe disease did not feel that their treatment was aggressive enough.(4)
Topical therapies
Topical therapies include emollients, keratolytics such as salicylic acid, corticosteroids, vitamin D analogues, tazarotene, tars, anthralin, tacrolimus and pimecrolimus.
Tacrolimus and pimecrolimus are particularly useful for facial and intertriginous psoriasis.(5) Daivobet ointment is a convenient, stable, once-daily fixed-combination product containing 0.5mg/g of betamethasone dipropionate and 50mg/g of calcipotriene. It has greater efficacy and a faster onset of action than its individual components or tacalcitol, a similar safety profile to that of betamethasone dipropionate, and is associated with 75% fewer cutaneous adverse events than tacalcitol and 50% less than calcipotriene.(6) Tazarotene is a receptor-selective retinoid that is typically used in combination with a topical corticosteroid or phototherapy. Enhanced efficacy is noted with combination therapy.(7) Tazarotene minimises epidermal atrophy induced by corticosteroids,(8) while the steroids minimise the irritancy of tazarotene.(9)
Phototherapy
Broadband (290–320nm) and narrow-band (311nm) ultraviolet B (UVB), psoralen + UVA (320–400nm) (PUVA) and sunlight (climatotherapy) are beneficial for patients with psoriasis. The use of narrow-band UVB is increasing, since its efficacy is greater than broadband UVB, approaching that of PUVA.(10) PUVA is used less frequently than in the past, since long-term treatment has been associated with squamous cell carcinoma; the risk of melanoma is controversial.(11) The 308nm excimer laser is safe and effective for localised plaques.(12)
Traditional systemic therapies
Methotrexate, acitretin and ciclosporin, which are the most widely prescribed systemic agents, require regular monitoring. In an effort to minimise total cumulative dosage and limit toxicity (eg, hepatotoxicity with methotrexate, nephrotoxicity with ciclosporin and skeletal effects with acitretin),(13) rotational therapy is commonly used.(14) With rotational therapy, patients are moved from one systemic agent to another or to phototherapy, either at set time intervals (eg, 1–2 years), when a flare occurs, when the drug loses efficacy, when toxicity occurs with a specific agent or when a toxic cumulative dose is approaching.
Biologicals
An increased understanding of the immunopathology of psoriasis has led to the development of more specific, targeted and safer therapies. Biological agents do not appear to be nephrotoxic or hepatotoxic, or to interfere with the metabolism of other medications.
Alefacept is a fusion protein consisting of leukocyte function-associated antigen type 3 (LFA-3) and human IgG1. It binds to CD2 on T-cells, interferes with T-cell activation and leads to apoptosis of memory (CD45RO+) T-cells.(15) Two weeks after 12 weeks of weekly intramuscular (IM) dosing, 21% of patients treated with 15mg alefacept achieved PASI-75 (psoriasis area and severity index reduction >-75%), compared with 5% of patients on placebo (p<0.001).(16) Greater improvement is noted after two courses of treatment.(15) Prolonged remissions have been noted following discontinuation of therapy. After one course of treatment, the median duration of remission (defined as >-PASI-50) was seven months in patients achieving a PASI-75.(15) Routine monitoring of lymphocyte subsets is required, since lymphopenia may occur.
Efalizumab is a humanised monoclonal antibody against the CD11a chain of LFA-l. It interferes with T-cell activation and trafficking into inflamed tissue. After 12 weeks of therapy, 27% of patients on efalizumab 1mg/kg/week subcutaneous (SC) achieved PASI-75, compared with 4% of patients on placebo (p<0.001). Flu-like symptoms may occur, primarily after the first and second doses. Flares of psoriasis after discontinuation and reversible thrombocytopenia have also been reported.(17)
Tumour necrosis alpha (TNFalpha) is elevated in psoriatic lesions(18) and in the synovial fluid in psoriatic arthritis.(19) TNFalpha blockers are very efficacious in the treatment of psoriasis and psoriatic arthritis.
Etanercept is a dimeric fusion protein containing the extracellular domain of the p75 TNF receptor fused to the Fc fragment of human IgG1. It binds to soluble and transmembrane-bound TNFalpha and lymphotoxin alpha.(20)
In a double-blind, randomised, placebo-controlled phase III study, PASI-75 was noted after 12 weeks of therapy in 34% of patients on 25mg SC twice weekly and in 49% of patients on 50mg twice weekly, compared with 4% of patients on placebo (p<0.001).(21) After 24 weeks of therapy, PASI-75 was achieved in 45% and 54% of patients, respectively.(22) Etanercept has also been shown to improve psoriatic arthritis and prevent radiographic progression.(23,24) The safety profile is good, with mild injection site reactions being the most common adverse events.(20) Rare cases of demyelinating disorders, opportunistic infections, lupus-like conditions and pancytopenia have been reported.(20)
Infliximab is a mouse–human chimeric monoclonal antibody that binds soluble and membrane-bound TNFalpha and triggers complement-dependent and cell-mediated cell lysis.(20) It is administered as a slow intravenous infusion. In a placebo-controlled study, 249 patients were dosed at weeks 0, 2 and 6.(25) At week 10, PASI-75 was obtained in 88% of patients treated with 5mg/kg, 72% treated with 3mg/kg and 18% on placebo (p<0.05). The most common reported adverse events were headache, pruritus, sinusitis and upper respiratory tract infections. Transfusion reactions are not uncommon.(20) Since long-term administration may be associated with neutralising antibodies, it is given in conjunction with methotrexate in patients with rheumatoid arthritis.(20) Rare cases of demyelinating disorders, opportunistic infections, including tuberculosis, and lupus-like conditions have been reported.(20) Infliximab is contraindicated in patients with severe infections or moderate-to-severe congestive heart failure.(26)
Conclusions
Topical treatment will remain the mainstay of treatment for localised disease. Combination therapy will likely expand with the development of new products. With the advent of biological agents, traditional systemic agents will be used less frequently. Biological agents are specific and targeted. They have been well studied and appear to be safe and efficacious. Long-term studies are required to determine whether there is a potential risk for malignancy.
References
- Clin Exp Dermatol 2001;26:314-20.
- J Cut Med Surg 2002;DOI:10.1007/s10227-002-0003-y.
- Guenther L, et al. Integrating biologic agents into management of moderate-to-severe psoriasis: a consensus of the Canadian Psoriasis expert panel [poster]. 10th International Psoriasis Symposium, Toronto, Canada; June 11–13, 2004.
- Arch Dermatol 2001;137:280-4.
- J Am Acad Dermatol 2003;48:564-8.
- Am J Clin Dermatol 2004;5:71-7.
- Am J Clin Dermatol 2003;4:197-202.
- Kaidbey K, et al. A pilot study to determine the effect of tazarotene 0.1% gel on steroid-induced epidermal atrophy [poster]. 58th Annual Meeting of the American Academy of Dermatology, San Francisco (CA, USA); 2000 March 10–15.
- J Am Acad Dermatol 1998;39:590-6.
- Exp Dermatol 2001;26:343-50.
- J Am Acad Dermatol 2004;50:613-22.
- J Am Acad Dermatol 2002;46:900-6.
- J Am Acad Dermatol 2001;45:649-61.
- J Am Acad Dermatol 1996;34:315-21.
- J Am Acad Dermatol 2002;47:821-33.
- J Eur Acad Dermatol Venerol 2003;17 Suppl 2:12-6.
- Leonardi CI, et al. The safety of efalizumab in patients with moderate to severe plaque psoriasis: summary of clinical trial experience [poster]. 62nd Annual Meeting of the American Academy of Dermatology, Washington (DC); 2004 February 6–11.
- Clin Exp Immunol 1994;96:146-51.
- J Rheumatol 1997;24:518-23.
- Arch Dermatol 2004;140:218-25.
- N Engl J Med 2003;349:2014-22.
- Efficacy and safety of etanercept in patients with psoriasis: results of a global phase III study [poster]. 62nd Annual Meeting of the American Academy of Dermatology, Washington (DC); 2004 February 6–11, 2004.
- Lancet 2000;356:385-90.
- Arthritis Rheum 2003;46 Suppl:S196.
- Gottlieb AB, et al. The efficacy of infliximab across a variety of subgroups with plaque psoriasis [poster]. 62nd Annual Meeting of the American Academy of Dermatology, Washington (DC); 2004 February 6–11.
- Compendium of pharmaceuticals and specialties. Repchinsky C, Welbanks L, Bisson R, et al, editors; Ottawa: Canada Pharmacists Association; 2004.
Resource
National Psoriasis Foundation
W:www.psoriasis.org/treatment/psoriasis
Events
European Academy of Dermatology and Venereology
Florence, Italy
17–20 November 2004
W:www.eadv.org
American Academy of Dermatology
New Orleans, LA
USA
18–22 February 2005
W:www.aad.org