The role of biosimilars in upholding quality patient care was explored during an Amgen Europe-sponsored satellite symposium at the 24th Congress of the European Association of Hospital Pharmacists (EAHP), Barcelona, Spain on 28 March 2019
Dr Gunar Stemer (Vienna General Hospital, Vienna) opened and chaired the symposium, with presentations from Dr Helen McBride (Amgen Biosimilars, USA), Professor João Gonçalves (University of Lisbon, Portugal) and Mr Simon Cheesman (University College London Hospitals NHS Foundation Trust, UK).
Supporting sustainable quality care
Global spending on medicines is increasing and the cost of biological medicines is a major driver for this increase:1 the overall global costs of medicines are projected to exceed US $1.5 trillion by 2023.1 Dr Gunar Stemer described three potential approaches to achieve sustainability in the face of limited healthcare resources: increase healthcare budgets, improve efficiency, and/or limit the medical offering. Of these options, improving efficiency is the most attractive.
Biosimilar medicines can play an important role in improving efficiency by driving cost savings and reducing ‘wasteful’ spending on more costly alternatives.2 As such, the potential savings from biosimilars – in addition to generic drugs – represent an important tool for achieving efficient and sustainable healthcare systems.2,3 The availability of biosimilars also has the potential to increase patient access to biological medicines (by reducing the cost of treatment) and release funds for new medicines as they come to market.2–4 While biosimilars can help to mitigate the explosion in cancer care costs, education is needed to correct any misperception of inferior quality compared with originator medicines and, therefore, support acceptance and maximum uptake.5
When selecting a biosimilar and evaluating which product offers the best value, criteria to consider include: production process/manufacturer; product specifications; clinical efficacy; and clinical safety and tolerability.6 For pharmacists in particular, further considerations include: ease of use/route of administration, vial size, storage considerations (including packaging and stability), supply chain reliability, support/education, reimbursement and price.6 Dr Stemer explained that in respect to the tendering process for biosimilars, a multi-tender/multiple ‘winner’ model may be preferable to a single tender/single ‘winner’ model in terms of overall cost (competition keeps price down) and, therefore, sustainability.3
Dr Stemer concluded by highlighting the role of hospital pharmacists in supporting sustainability of care by ensuring responsible, criteria-based product selection, providing education, and safeguarding the onboarding of biosimilars. Sustainable care encompasses multiple factors including patient access to quality medicines, efficient use of healthcare budgets, and healthy levels of supply and competition.3 Ultimately, it must meet the needs of all stakeholders including patients, healthcare professionals/providers, payers and manufacturers (Figure 1).3
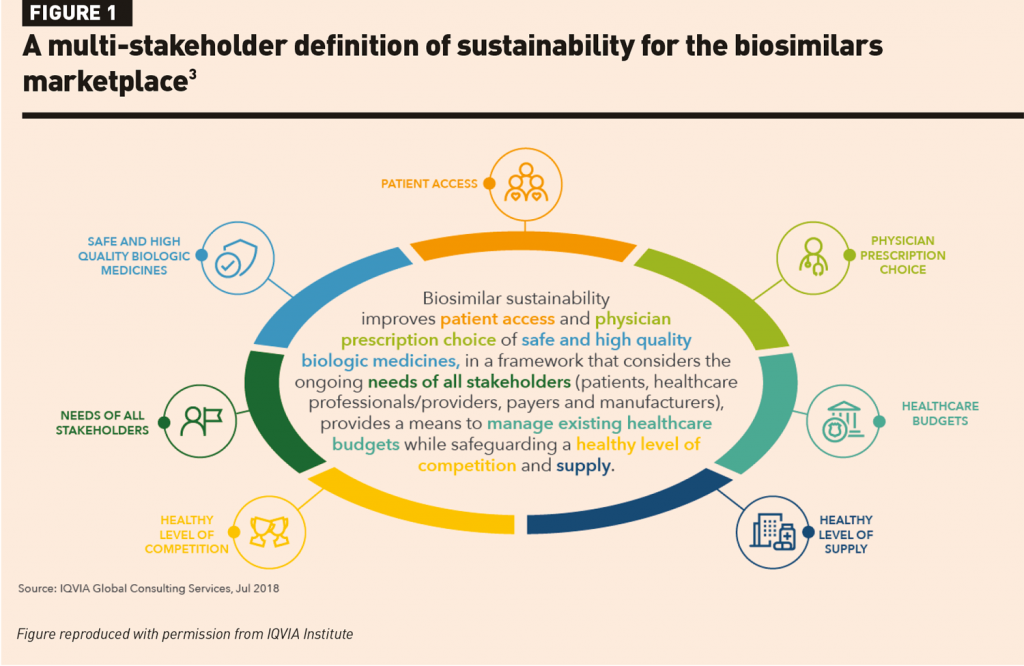
Pathway to the pharmacy: critical quality attributes
Dr Helen McBride began by summarising the differences between biologics and chemically synthesised generic drugs, biologics being larger and structurally more complex.7–9 The complexity of biologics, particularly monoclonal antibodies, is reflected in the potential for post-translational modifications that can, in turn, affect mechanism of action, bioavailability, clearance, immunogenicity, effector function and binding.10,11 Manufacturing of biological products is a proprietary multistep process, with each manufacturer using proprietary techniques and unique cell lines.10,12 Post-translational modifications can be influenced by the manufacturing process (for example, cell type and culture conditions, purification, and raw materials)13,14 and hence the manufacturing process must be tightly controlled to ensure product consistency and quality over time.13,15,16
Biosimilar development is performed via a stepwise approach to demonstrate biosimilarity and bioequivalence to the reference product.10,12 The goal of biosimilar development is to demonstrate clinical equivalence, not to independently re-establish safety and efficacy which have already been extensively studied in clinical trials of the reference product (Figure 2).16–18 Analytical characterisation forms the foundation of biosimilar development, establishing similarity of both structure and function to that of the reference product.16–18 Biosimilar manufacturers start with limited knowledge of the reference product; therefore, biosimilars are developed based on information from testing the reference product and establishing a list of critical quality attributes (CQAs) that need to be matched within an expected quality range (Figure 3).12,16–19 CQAs are specific attributes that can impact biologic activity, such as potency, pharmacokinetics (PK) and immunogenicity.12
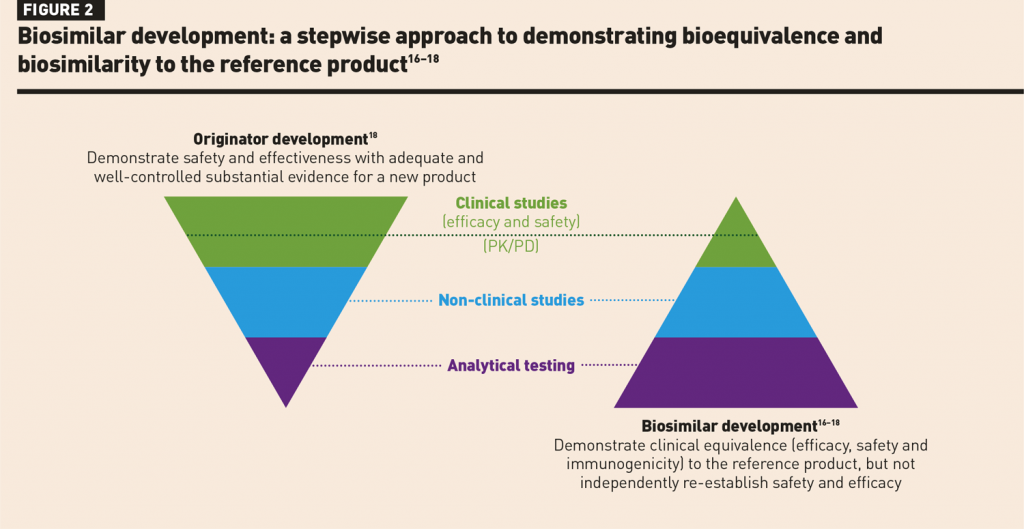
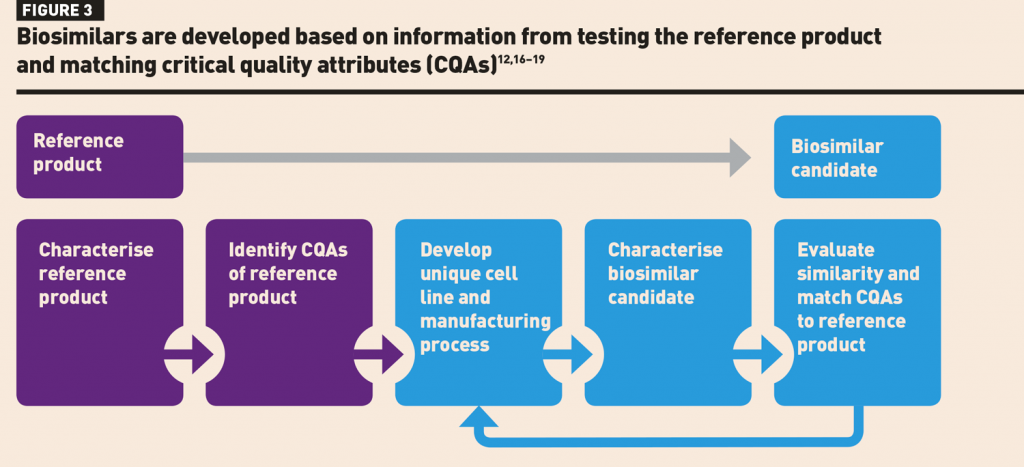
While the analytical characterisation phase of biosimilar development aims to match both the structure and function of the candidate biosimilar to the reference product, minor structural differences are acceptable provided they do not impact function.19,20 If structural comparisons leave residual uncertainties, functional studies are the first step in addressing these. Dr McBride presented an example of a candidate biosimilar product which fell outside of the defined glycosylation quality range during structural analysis. However, functional testing associated with glycosylation showed that the biosimilar remained within the defined functional quality range.20 Another challenge that biosimilar manufacturers may face is an observed shift in the structure and/or function of the reference product, for example due to manufacturing changes. This was encountered during the development of rituximab and trastuzumab biosimilars.21,22
Dr McBride concluded that analytical characterisation, including identifying and establishing similarity in CQAs for the biosimilar candidate and reference product, forms the foundation of biosimilar development. However, it must be considered as part of the whole totality of evidence – comprising analytical characterisation, non-clinical studies and clinical studies (PK/pharmacodynamics [PD], efficacy and safety) − in order to justify and confirm biosimilarity and clinical equivalence.16–18
Monoclonal antibodies in the pharmacy: practical considerations
Professor João Gonçalves started his presentation by noting how the extensive analytical and quality characterisation that is required during biosimilar development has not only given confidence in biosimilar use but has also provided important insights that can be applied to biologics more broadly. Such insights have influenced the European Medicine Agency’s ‘Guideline on good pharmacovigilance practices’.23 During the development of a trastuzumab biosimilar, for example, shifts in antibody-dependent cellular toxicity (ADCC) were observed in the trastuzumab reference product; these shifts were associated with manufacturing changes, highlighting the importance of closely monitoring product specifications following such changes.22 Other findings have emphasised how product quality is influenced by multiple factors that pharmacists should be aware of. During the development of an infliximab biosimilar, reductions in protein concentration were observed in batches of the reference product that were nearing their expiry date,24 reflecting the fact that storage has an influence on quality, with a decay in protein concentration occurring over time for most biologics.
Assessment of immunogenicity is a key part of biosimilar clinical development,16–18 with the risk of anti-drug antibody formation varying depending on the protein evaluated.25 Despite increased sensitivity of the assays that are currently used to identify anti-drug antibodies, there is no evidence that the risk of immunogenicity is greater with biosimilars compared with their reference products.26 However, during product handling and preparation, there are a number of factors which can influence biologic quality, in particular with regard to risk of immunogenicity. These factors include changes in temperature, the addition of diluent, and the impact of mechanical stresses such as shaking, any of which may result in changes such as protein aggregation or precipitation. For example, agitation in the presence of silicone oil may accelerate protein aggregation, which may, in turn, stimulate the development of anti-drug antibodies.27 Particularly for products such as adalimumab and infliximab which have been associated with a higher overall immunogenic risk, the development of neutralising anti-drug antibodies may lead to reductions in effective drug plasma levels and be a potential cause of therapeutic failure.28 Professor Gonçalves suggested that pharmacists are ideally placed to act as guardians of biologic product quality in the hospital. In particular, to validate the use of biosimilars as used in routine clinical practice, pharmacists should be involved in monitoring immunogenicity and drug trough plasma levels following the switch to a biosimilar, to provide reassurance that quality is maintained during standard handling procedures.
In summary, Professor Gonçalves noted that ensuring drug quality does not finish when a product is released by the manufacturer − quality needs to be maintained at all points until the product is administered to the patient. Pharmacists play a critical role in maintaining quality systems and pharmacovigilance monitoring in the clinic.
Integrating biosimilars into practice
While biosimilars have the potential to make a significant contribution to the sustainability of healthcare systems, a number of barriers can limit their uptake in clinical practice. Mr Simon Cheesman described how pharmacists have a pivotal role in overcoming these barriers and in maximising the potential cost savings associated with their use.
Switching to a biosimilar takes time and effort. The initial process of selecting from a range of potential biological products is not always simple and straightforward. In the UK, the focus has been on identifying the ‘best value’ biologic which considers multiple factors − in addition to cost alone – such as clinical trial data, reliability of supply, and other attributes such as the range of vial sizes or administration devices available (Figure 4). Of note, the ‘best value’ biologic may be either a biosimilar or the reference product. Pharmacists need to be aware of the range of products available and their accompanying data packages and attributes in order to inform selection discussions.
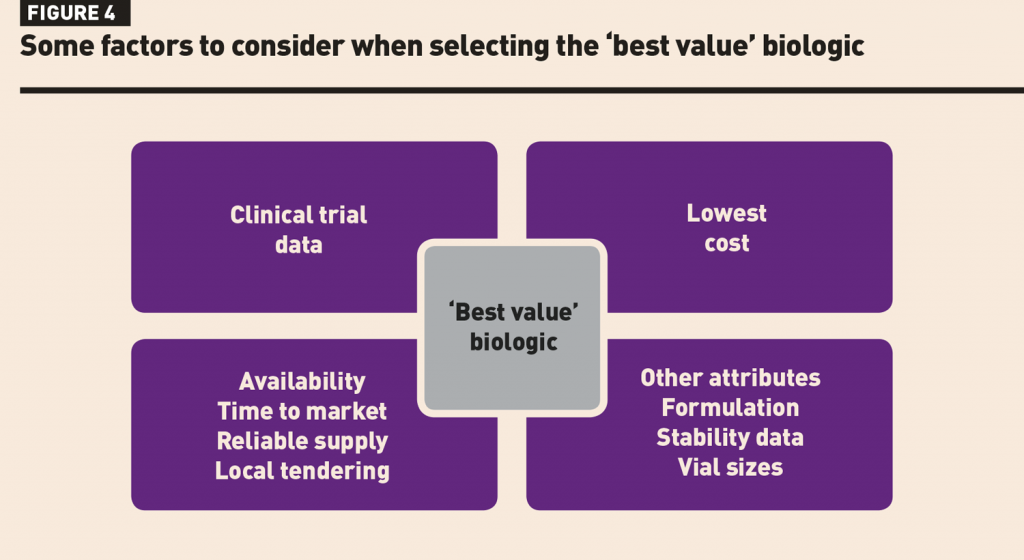
When introducing a biosimilar, there are many stakeholders to consult, including clinicians, nurses and patients. Among clinicians, a spectrum of acceptance of biosimilars − ranging from reluctance to acceptance to enthusiasm − may be seen. The pharmacist’s aim should be to help clinicians achieve a position of acceptance where they feel comfortable with biosimilar use and are equipped to address patient queries that may arise. Where there is reluctance, this can often be addressed through education, particularly around the biosimilar approval process and principles such as extrapolation. The results of a study performed by Mr Cheesman’s centre confirmed that a pharmacist-driven education programme involving a short training session on biosimilars was associated with increased understanding and confidence in biosimilars compared with knowledge prior to the training.29 Regarding patient acceptance, ensuring transparency of switching and providing an opportunity to ask questions is important. Mr Cheesman noted that prior to switching to a biosimilar, patients frequently ask questions such as ‘What does my doctor think?’ thus highlighting the importance of both physician education and of the ‘one voice’ concept where patients receive consistent information from members of their multidisciplinary healthcare team.
The generation of real-world data on biosimilars can support confidence in their use among healthcare professionals. In addition, there may be specific approaches to use of the originator product for which real-world data on the biosimilar may be particularly beneficial. An example is data on rapid infusion of intravenous rituximab, which is common for the originator product.30 Reverting to a more prolonged infusion with a biosimilar could present challenges, for example, reductions in day care unit capacity and a diminished patient experience. Mr Cheesman described a study which demonstrated that rapid infusion of biosimilar rituximab – as is standard practice for the originator – was not associated with an increase in the rate of infusion-related reactions, and that there was no need to re-escalate the infusion rate for switching patients.31 In summary, the uptake of biosimilars is rapidly increasing as confidence in them grows and, with pharmacists’ input, large cost savings are achievable without compromising patient outcomes or experience.
Concluding remarks
Overall, the symposium highlighted that biosimilars provide an important tool to achieve sustainability of quality patient care in a landscape of increasing healthcare costs. Hospital pharmacists play a key role in supporting biosimilar uptake and acceptance by other healthcare professionals; therefore, it is important that they are knowledgeable about the rigorous biosimilar development process, practical considerations, and strategies for effective biosimilar implementation into clinical care.
• This report summarises presentations given by the speakers at an Amgen Europe-sponsored satellite symposium held at the 24th Congress of the EAHP, Barcelona, Spain on 28 March 2019. The publication of this article was funded by Amgen Europe and writing assistance (drafting the report) was provided by Isabel Miller, Elements Communications Ltd, Westerham, UK, funded by Amgen Europe. The views and opinions reflect those expressed by the speakers and are not necessarily those of Amgen.
References
1 IQVIA The Global Use of Medicine in 2019 and Outlook to 2023 Report, January 2019. www.iqvia.com/institute/reports/the-global-use-of-medicine-in-2019-and-outlook-to-2023 (accessed April 2019).
2 OECD/EU (2018). Health at a Glance: Europe 2018: State of Health in the EU Cycle, OECD Publishing, Paris. https://ec.europa.eu/health/sites/health/files/state/docs/2018_healthatglance_rep_en.pdf (accessed April 2019).
3 IQVIA Advancing Biosimilar Sustainability in Europe – A Multi-Stakeholder Assessment, September 2018. https://www.iqvia.com/institute/reports/advancing-biosimilar-sustainability-in-europe (accessed April 2019).
4 McCamish M, et al. Worldwide experience with biosimilar development. MAbs 2011;3:209–17.
5 Pricing of cancer medicines and its impacts. Geneva: WHO; 2018. Licence: CC BY-NC-SA 3.0 IGO. https://apps.who.int/iris/bitstream/handle/10665/277190/9789241515115-eng.pdf?ua=1 (accessed April 2019).
6 Boone N et al. How to select a biosimilar. Eur J Hosp Pharm 2013;20:275–86.

7 EMA. Biosimilar medicines: overview. www.ema.europa.eu/en/human-regulatory/overview/biosimilar-medicines-overview (accessed April 2019).
8 Genazzani AA et al. Biosimilar drugs: concerns and opportunities. BioDrugs 2007;21:351–6.
9 Lipman NS et al. Monoclonal versus polyclonal antibodies: Distinguishing characteristics, applications, and information resources. ILAR J 2005;46:258–68.
10 Roger SD. Biosimilars: how similar or dissimilar are they? Nephrology (Carlton) 2006;11:341–6.
11 Dörner T, Kay J. Biosimilars in rheumatology: current perspectives and lessons learnt. Nat Rev Rheumatol 2015;11: 713–24.
12 Vulto AG, Jaquez OA. The process defines the product: what really matters in biosimilar design and production? Rheumatology 2017;56:iv14–iv49.
13 Declerck P et al. Biosimilarity versus manufacturing change: two distinct concepts. Pharm Res 2016;33:261–8.
14 Grampp G et al. The diversity of biosimilar design and development: implications for policies and stakeholders. BioDrugs 2013;27:305–16.
15 Blauvelt A et al. Biosimilars for psoriasis: preclinical analytical assessment to determine similarity. Br J Dermatol 2016;174:282–6.
16 EMA. Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: quality issues (revision 1), 2014.
17 EMA. Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: non-clinical and clinical issues, 2012.
18 FDA. Scientific considerations in demonstrating biosimilarity to a reference product. Guidance for industry, 2015.
19 ICH Harmonised Tripartite Guideline. Comparability of biotechnological/biological products subject to changes in their manufacturing process Q5E, 2004.
20 Tsong Y et al. Development of statistical methods for analytical similarity assessment. J Biopharm Stat 2017;27:197–205.
21 Visser J et al. Physicochemical and functional comparability between the proposed biosimilar rituximab GP2013 and originator rituximab. BioDrugs 2013;27:495–507.
22 Kim S et al. Drifts in ADCC-related quality attributes of Herceptin®: Impact on development of a trastuzumab biosimilar. mAbs 2017;9:704–14.
23 Guideline on good pharmacovigilance practices (GVP) product- or population-specific considerations II: biological medicinal products. https://www.ema.europa.eu/en/human-regulatory/post-authorisation/pharmacovigilance/good-pharmacovigilance-practices (accessed April 2019).
24 REMSIMA® (infliximab) CHMP Assessment Report: EMA/CHMP/589317/2013. June 2013.
25 Immunogenic potential scale. http://epivax.com/blog/plosone (accessed April 2019).
26 Cohen HP et al. Switching reference medicines to biosimilars: a systematic literature review of clinical outcomes. Drugs 2018;78:463–78.
27 Thirumangalathu R, et al. Silicone oil- and agitation-induced aggregation of a monoclonal antibody in aqueous solution. J Pharm Sci 2009;98:3167–81.
28 Dreesen E et al. Practical recommendations for the use of therapeutic drug monitoring of biopharmaceuticals in inflammatory diseases. Clin Pharmacol 2017;9:101–11.
29 Murphy P et al. Impact of education programme on biosimilar attitudes and beliefs. BOPA 2017; Abstract 66.
30 Sehn LH et al. Rapid infusion rituximab in combination with corticosteroid-containing chemotherapy or as maintenance therapy is well tolerated and can safely be delivered in the community setting. Blood 2007;109:4171–3.
31 Shah R et al. Evaluation of the safety and tolerability of rapid infusion of biosimilar rituximab Truxima® – the University College London Hospitals (UCLH) Experience. ASH Annual Meeting 2017; Abstract 3387.

