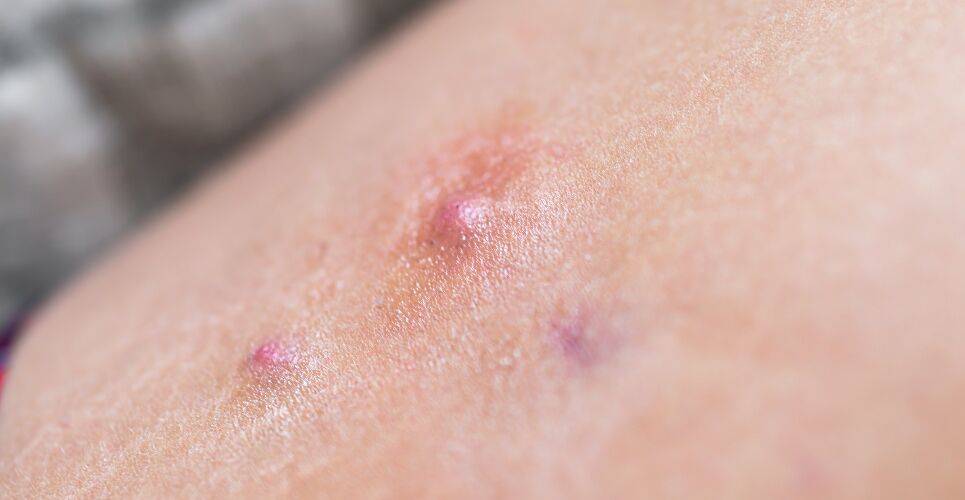
Bimekizumab (brand name Bimzelx) has been granted marketing authorisation by the European Commission (EC) for active moderate-to-severe hidradenitis suppurativa (HS), its manufacturer UCB has announced.
Now indicated for adults with an inadequate response to conventional systemic HS therapy, the humanised monoclonal IgG1 antibody is designed to selectively inhibit the two cytokines interleukin 17A (IL-17A) and interleukin 17F (IL-17F).
This approval makes bimekizumab the first biologic for moderate-to-severe HS to target IL-17F in addition to IL-17A.
It also marks the fourth marketing authorisation for the drug, adding to the existing indications in moderate-to-severe plaque psoriasis, active psoriatic arthritis and active axial spondyloarthritis.
Professor Christos Zouboulis, director of the departments of dermatology, venereology, allergology and immunology at Städtisches Klinikum Dessau, Brandenburg Medical School, Germany, and president of the European Hidradenitis Suppurativa Foundation, said: ‘The European Commission’s approval of bimekizumab marks a significant milestone for the EU hidradenitis suppurativa community, particularly considering the limited treatment options currently available.
‘As a community, we strive to improve the management of hidradenitis suppurativa. Bimekizumab offers a promising new therapeutic option for moderate to severe disease, supported by Phase 3 evidence that demonstrated clinically meaningful and sustained responses over 48 weeks.’
Indeed, the EC approval was based on results from the BE HEARD I and BE HEARD II studies, which evaluated the efficacy and safety of bimekizumab in the treatment of moderate-to-severe HS.
Clinically meaningful improvements with bimekizumab
The multicentre, randomised, double-blind, placebo-controlled phase 3 trials had a combined enrolment of 1,014 adult patients with a diagnosis of moderate to severe HS.
The primary endpoint in both studies was HiSCR50 at Week 16, with HiSCR75 at Week 16 as a key secondary endpoint. These are defined as at least either a 50 or 75% reduction from baseline in the total abscess and inflammatory nodule count, with no increase from baseline in abscess or draining tunnel count.
A significantly higher proportion of patients treated with bimekizumab versus placebo achieved a 50% or greater improvement in HS signs and symptoms at Week 16, as measured by HiSCR50.
Bimekizumab treatment also resulted in clinically meaningful improvements in the ranked key secondary endpoint, HiSCR75 versus placebo at Week 16.
Responses were maintained to Week 48, and the safety profile of bimekizumab was consistent with safety data seen in previous trials with no new observed safety signals.