teaser
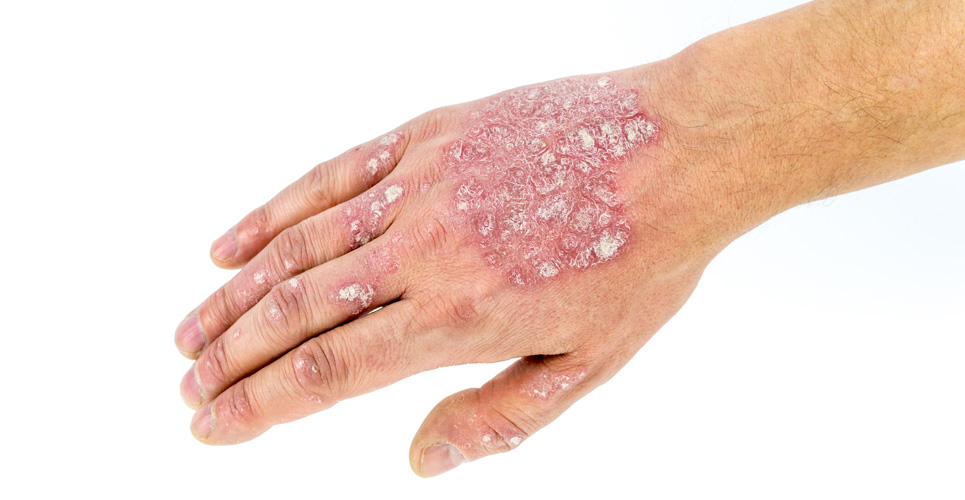
Safety data from 10 years of clinical development and postmarketing experience in patients treated with Raptiva (efalizumab), presented at the European Academy of Dermatology and Venereology (EADV) congress, have confirmed the favourable safety profile of Raptiva in long-term treatment of patients with moderate to severe chronic plaque psoriasis.
There is a significant unmet clinical need for efficacious treatments that can be used safely in the long-term to effectively manage this life long condition and help prevent the damaging effects of comorbidities that result from poorly controlled psoriasis.
As of April 2007, more than 40,000 patients have received Raptiva corresponding to a cumulative clinical experience of >28,000 patient-years of exposure and data continues to be collected for future investigation.
These data, collected over a decade from experience during clinical development and post-marketing surveillance, were gathered and a safety review carried out. Results demonstrate Raptiva’s favourable safety profile and show that rates of known adverse events have remained stable throughout the post-marketing period.
In addition to this positive safety data, results of the longest continuous study of any biological therapy for psoriasis treatment were published in the British Journal of Dermatology in May 2008.
Professor Kim Papp, Founder and President of Probity Medical Research said “Chronic inflammatory systemic diseases like psoriasis require long-term treatment and care. These data are very encouraging and provide support for efalizumab as an appropriate treatment option for many patients with moderate to severe plaque psoriasis.”
“Patients with moderate to severe psoriasis require life-long management of their disease,” said Professor Mehmet Ali Gurer, EADV Spring Symposium Chairman and Professor of Dermatology, Gazi University Medical School, Ankara, Turkey.
“Recent findings around reduced life expectancy and comorbidities suggest that there should be shift towards treating patients earlier and more aggressively to control the chronic systemic inflammation.
“This requires a treatment that is convenient and appropriate for continuous use with a favourable safety profile over the long-term. Raptiva, which is a T cell modulator, has a unique and targeted mode of action (MoA) which provides enduring disease reduction up to three years.”